Chapter 4 Atomic Structure Early Atomic Theory n








- Slides: 18

Chapter 4 Atomic Structure

Early Atomic Theory n n Greeks: The world is made of two things, empty space and “atoms”. Atoms are the smallest possible stuff. Aristotle: Matter is continuous, it isn’t made from smaller stuff.

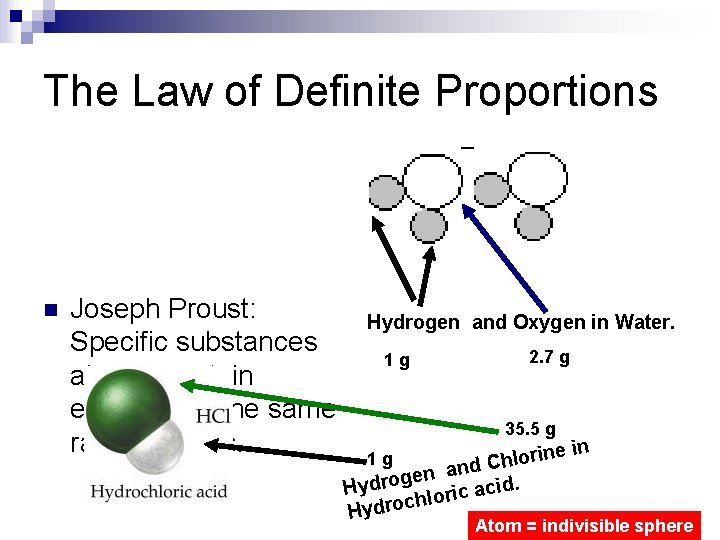
The Law of Definite Proportions n Joseph Proust: Specific substances always contain elements in the same ratio by mass. Hydrogen and Oxygen in Water. 1 g 2. 7 g 35. 5 g rine o l h C and n e g o cid. Hydr a c i r o chl Hydro 1 g in Atom = indivisible sphere

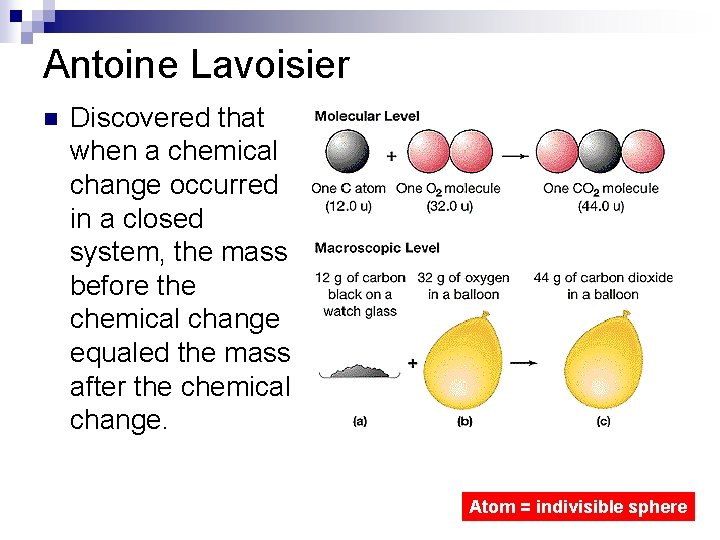
Antoine Lavoisier n Discovered that when a chemical change occurred in a closed system, the mass before the chemical change equaled the mass after the chemical change. Atom = indivisible sphere

Dalton’s Atomic Theory Elements are made of tiny particles called atoms. n All atoms of a given element are identical. n All atoms of a given element are different from the atoms of other elements. n Atoms can combine with each (in small whole number ratios) other to form different compounds. (ex: NO, NO 2, N 2 O) n Atoms can are indivisible. be divided. Electrons are n transferred during chemical reactions, and nuclear reactions are possible, though rare. Atom = indivisible sphere

JJ Thomson presented three hypotheses about cathode rays based on his 1897 experiments: n He discovered that particles could come from atoms which had a negative charge, and bent in response to a magnet. n n http: //www. aip. org/history/electron/jj 1897. htm Atom = sphere containing negative charges

Robert Millikan n Oil Drop Experiment n Millikin sprayed oil through an atomizer, as it exits the chamber it becomes negatively charged. The bottom plate is negative, and repels the negatively charged oil droplet, so it hovers above the bottom plate if there is enough negative charge to repel the drop. By adjusting the amount of charge, Millikan found that the charges on the doplets were a multiple of the same number. y = mx ; where the multiple was “m” is the amount of charge = to one charge. “X” was the independent variable in this experiment. n n Atom = sphere containing negative charges

Henry Mosely studied X-rays from anodes of different metals. n The wavelength (color) produced by these X-rays depends on the number of protons. n The number of protons = atomic number. n Z = atomic number. n Atomic number determines the identity of the atom. Atom = sphere containing n both positive and negative charges

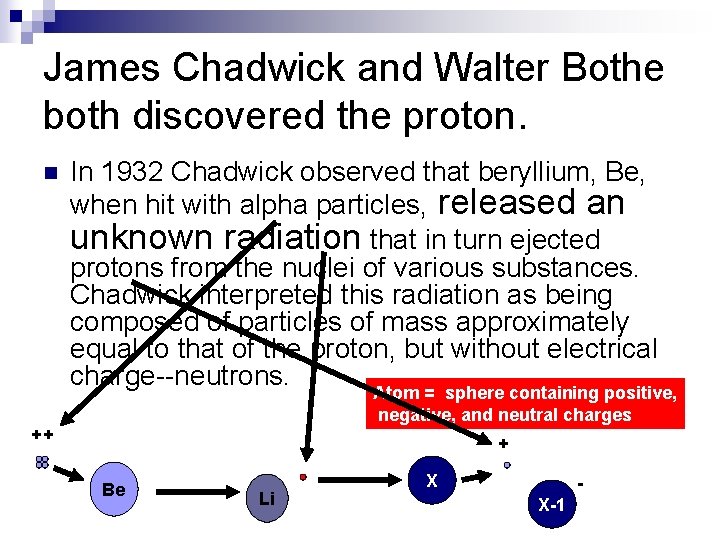
James Chadwick and Walter Bothe both discovered the proton. n In 1932 Chadwick observed that beryllium, Be, when hit with alpha particles, released an unknown radiation that in turn ejected protons from the nuclei of various substances. Chadwick interpreted this radiation as being composed of particles of mass approximately equal to that of the proton, but without electrical charge--neutrons. Atom = sphere containing positive, negative, and neutral charges ++ + Be Li X X-1

Isotopes: J. J. Thomson observed two kinds of neon atoms. neon-20 and neon-21 n Both neons are chemically identical, but their masses differ. n Atom = sphere containing positive, negative charges, and neutral particles.

Henry Moseley 1913 Using X-rays, Moseley discovered that the wavelength of X-rays is characteristic of the metal used as an anode. n The number of protons = the identity of the element. n Atom = sphere containing positive, negative charges, and some number of neutral particles, depending upon the isotope.

Summary n n n n n Democritus: Atom Aristotle: Continuous Matter Proust: the Law of Definite Composition Lavosier: the Law of Conservation of Matter Dalton: “Modern” Atomic Theory JJ Thompson: Discovery of Electrons Millikin: Charge/Mass ratio of electrons Moseley: Discovery of Protons/Atomic Number Chadwick and Bothe: Discovery of Neutrons

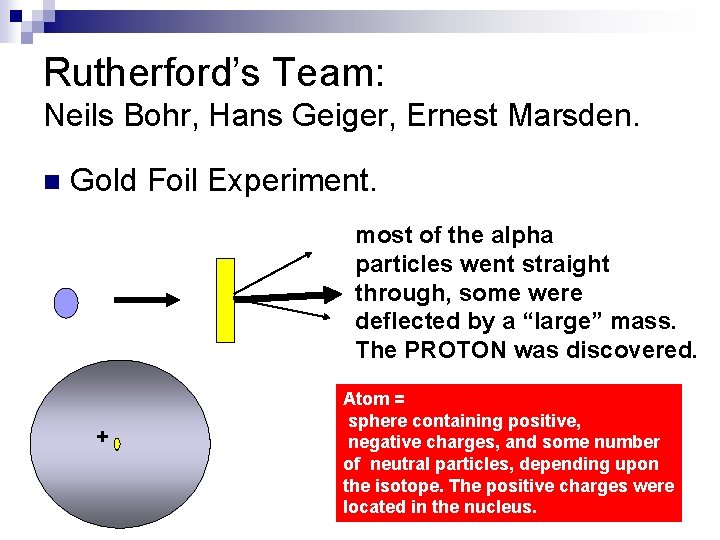
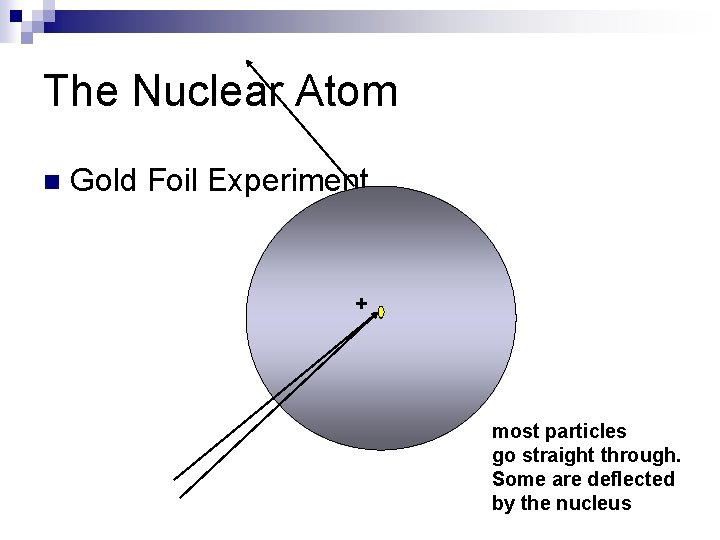
Rutherford’s Team: Neils Bohr, Hans Geiger, Ernest Marsden. n Gold Foil Experiment. most of the alpha particles went straight through, some were deflected by a “large” mass. The PROTON was discovered. + Atom = sphere containing positive, negative charges, and some number of neutral particles, depending upon the isotope. The positive charges were located in the nucleus.

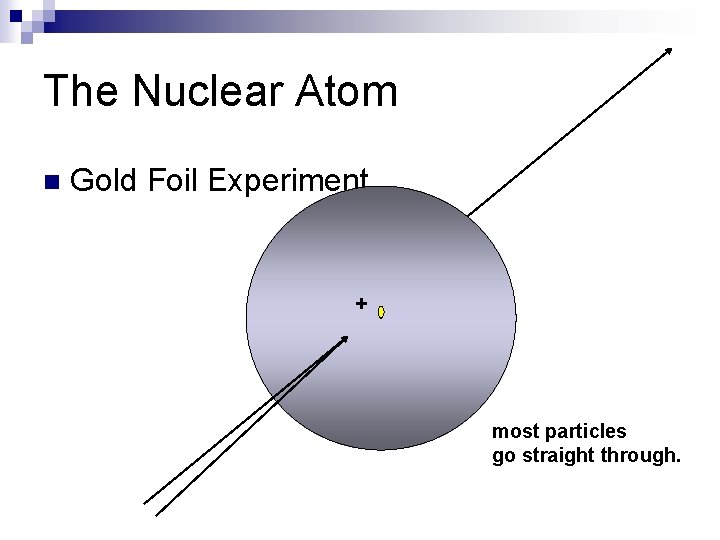
The Nuclear Atom n Gold Foil Experiment. + most particles go straight through.

The Nuclear Atom n Gold Foil Experiment. + most particles go straight through. Some are deflected by the nucleus

Summary n n n n n Democritus: Atom Aristotle: Continuous Matter Proust: the Law of Definite Composition Lavosier: the Law of Conservation of Matter Dalton: “Modern” Atomic Theory JJ Thompson: Discovery of Electrons Millikin: Charge/Mass ratio of electrons Moseley: Discovery of Protons/Atomic Number Chadwick and Bothe: Discovery of Neutrons Rutherford: The Nuclear Atom

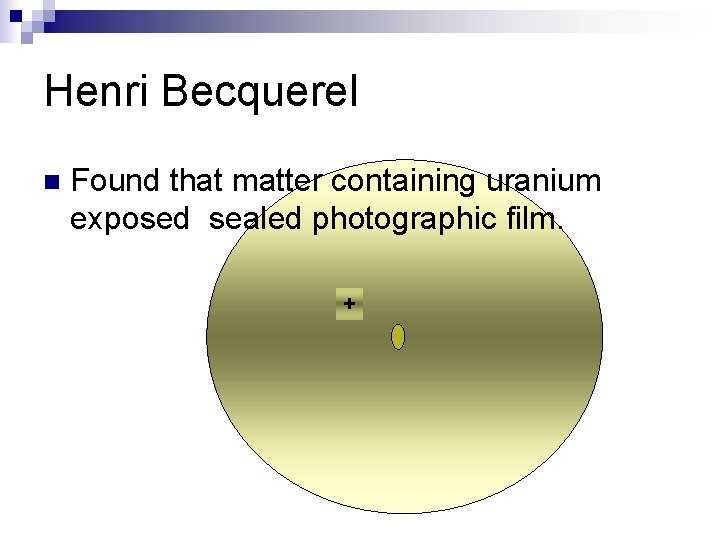
Henri Becquerel n Found that matter containing uranium exposed sealed photographic film. +

Time-Line of Discovery http: //www. chemheritage. org/explore/matt er-time. html n Other web sites n n n http: //www. slcc. edu/schools/hum_sci/physics/whatis/biography/dalton. html http: //www. chemheritage. org/Educational. Services/chemach/ppt/jd. html http: //antoine. frostburg. edu/chem/senese/101/atoms/dalton. shtml http: //www. fact-index. com/l/la/law_of_definite_proportions. html