Writing Names and Formulas for Compounds Naming Ametal
















































- Slides: 70

Writing Names and Formulas for Compounds

Naming A-metal compounds Rules for naming A-metal compounds: Ex. Li. F 1) Write the name of the metal (lithium) 2) write the name of the non-metal, changing the ending to –ide (fluoride) The name would be lithium fluoride

A-metal practice Mg. Cl 2 Na. Br Al. N K 3 N Ca 3 P 2 magnesium chloride sodium bromide aluminum nitride potassium nitride calcium phosphide

All non-metals and their proper -ide ending nitride oxide fluoride phosphide sulfide chloride arsenide selenide bromide telluride iodide astatide

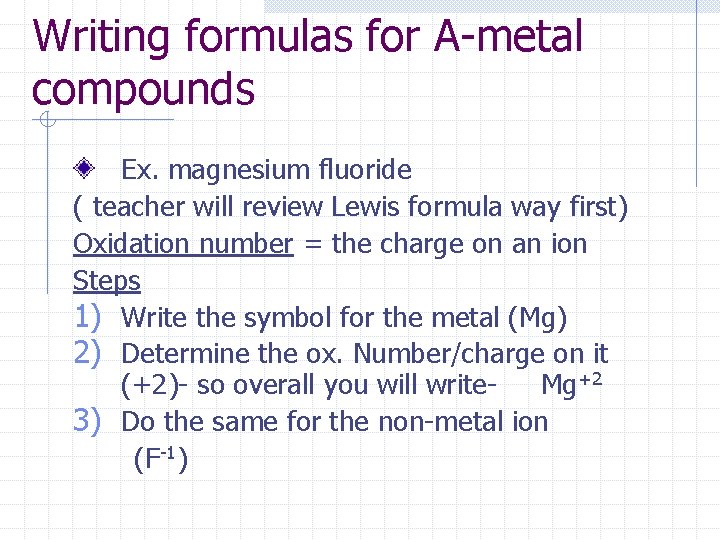
Writing formulas for A-metal compounds Ex. magnesium fluoride ( teacher will review Lewis formula way first) Oxidation number = the charge on an ion Steps 1) Write the symbol for the metal (Mg) 2) Determine the ox. Number/charge on it (+2)- so overall you will write. Mg+2 3) Do the same for the non-metal ion (F-1)

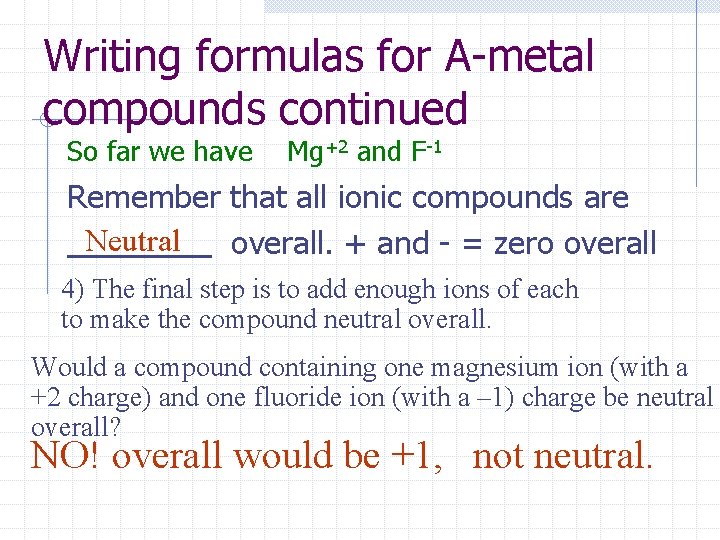
Writing formulas for A-metal compounds continued So far we have Mg+2 and F-1 Remember that all ionic compounds are Neutral overall. + and - = zero overall 4) The final step is to add enough ions of each to make the compound neutral overall. Would a compound containing one magnesium ion (with a +2 charge) and one fluoride ion (with a – 1) charge be neutral overall? NO! overall would be +1, not neutral.

Writing formulas for A-metal compounds continued Mg+2 and F-1 - this is where we left off How many fluoride ions would the formula need to be neutral overall? TWO! Thus the formula should be… Mg. F 2 Mg+2 F-1 = 0

Writing formulas for A-metal Let’s Try Another Ex. aluminum bromide Write the symbols: Al Br Determine the charges: Al+3 Br -1 Add enough of both ions to make it neutral What will the formula be? Al. Br 3

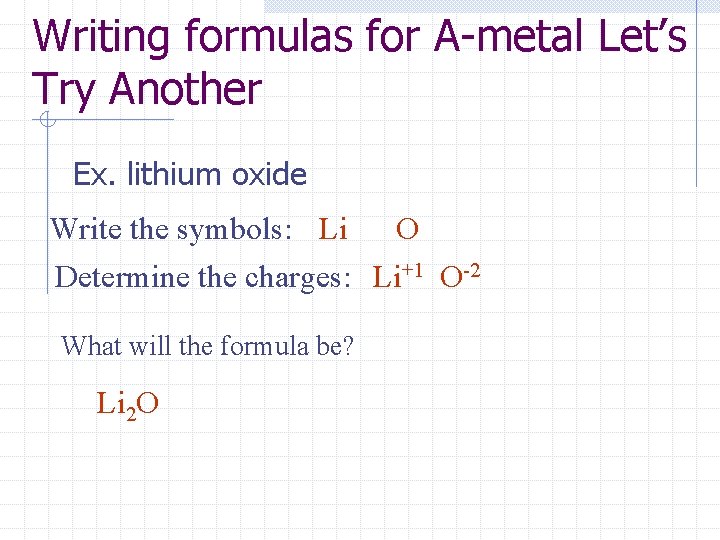
Writing formulas for A-metal Let’s Try Another Ex. lithium oxide Write the symbols: Li O Determine the charges: Li+1 O-2 What will the formula be? Li 2 O

Shortcut SOXS Symbols Oxidation # Criss-cross Simplify!

Practice writing formula of Ametal compounds Try these on your own 1) calcium oxide 2) calcium fluoride 3) sodium nitride

Practice answers Ex. calcium oxide Symbols with charges: Ca+2 O-2 What will the formula be? Ca. O

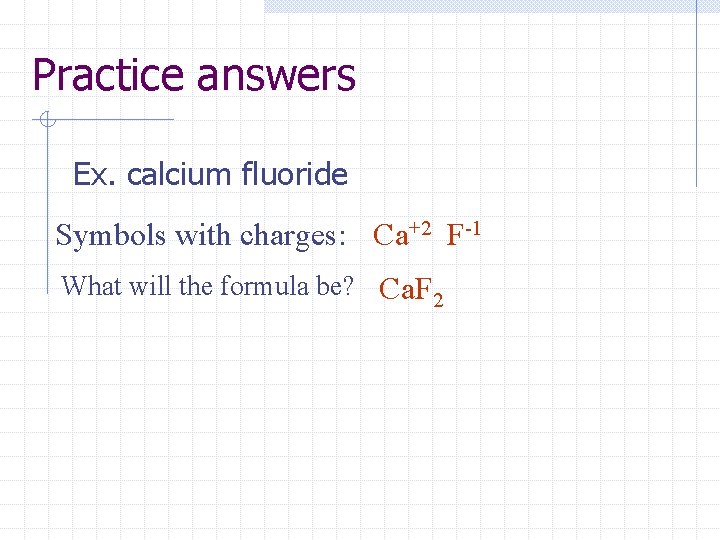
Practice answers Ex. calcium fluoride Symbols with charges: Ca+2 F-1 What will the formula be? Ca. F 2

Practice answers Ex. sodium nitride Symbols with charges: Na+1 N-3 What will the formula be? Na 3 N

Try this one- it’s tougher Ex. aluminum oxide Symbols with charges: Al+3 O-2 What will the formula be? Hint: more than one of each is needed. Try having two of the odd charged ion. (Al+3 +3 is the odd charge) Al+3= +6 charge total: -6 is now needed O-2 O-2 = -6 charge +6 -6=0 Thus the formula is Al 2 O 3

Another tough one. . Ex. Magnesium phosphide Symbols with charges: Mg+2 P-3 What will the formula be? Try starting with two of the odd charge. P-3 = -6 Mg+2 = +6 Thus the formula is Mg 3 P 2

Let’s finish up A-metals… Try these tough ones on your own 1) beryllium nitride 2) aluminum sulfide 3) strontium arsenide

Practice answers Ex. beryllium nitride Symbols with charges: Be+2 N-3 What will the formula be? Be 3 N 2

Practice answers Ex. aluminum sulfide Symbols with charges: Al+3 S-2 What will the formula be? Al 2 S 3

Practice answers Ex. strontium arsenide Symbols with charges: Sr+2 As-3 What will the formula be? Sr 3 As 2

Naming Compounds when given the formula Jump to T-metals Activity Go forward to Tmetal before this activity

Writing Formulas activity Yellow - Blue - White cards

Formulas Remember, all ionic compounds are neutral overall. Neutral!!!!!! Throughout this activity you must remember this

Writing Formulas: A-metals Lithium Fluoride Sodium chloride Magnesium oxide Calcium Fluoride Magnesium chloride Aluminum fluoride Lithium nitride

Strontium sulfide francium telluride Aluminum iodide Beryllium phosphide Cesium nitride Potassium iodide Sodium selenide

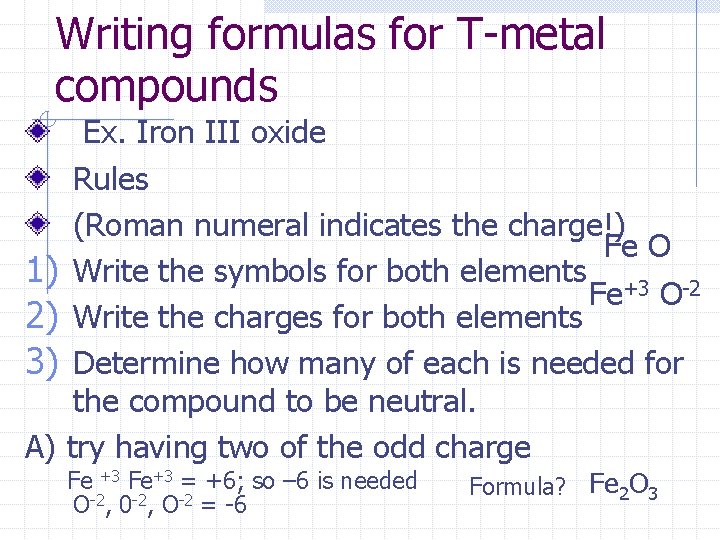
Writing formulas for T-metal compounds Ex. Iron III oxide Rules (Roman numeral indicates the charge!) Fe O 1) Write the symbols for both elements +3 -2 Fe O 2) Write the charges for both elements 3) Determine how many of each is needed for the compound to be neutral. A) try having two of the odd charge Fe +3 Fe+3 = +6; so – 6 is needed O-2, 0 -2, O-2 = -6 Formula? Fe 2 O 3

Practice Writing formulas for T -metals Titanium II oxide Write symbols with charges: Determine formula: Ti+2 O-2 Ti. O Cobalt IV oxide: Symbols/charges: Co Formula? +4 Co. O 2 O -2

Writing Formulas- T-metals Copper I chloride Copper II chloride Titanium II fluoride Titanium IV Fluoride Iron II oxide Iron III nitride Copper I Sulfide

Writing Formulas- tougher ones Calcium phosphide Magnesium nitride Aluminum sulfide Iron III oxide (rust) Titanium II oxide Titanium IV sulfide Titanium IV phosphide

Writing Names Activity Pink-Green- White cards

Writing names: A-metals Li. F Na 2 S Li. Cl Ca. O Al 2 S 3 Mg 3 P 2 lithium fluoride

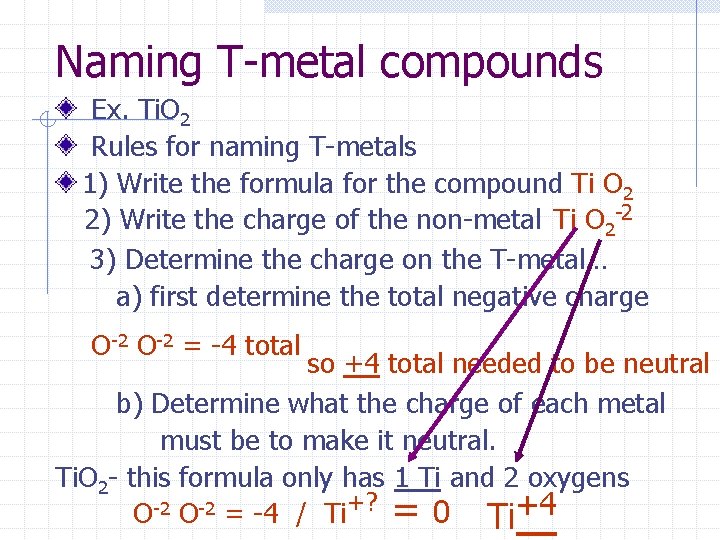
Naming T-metal compounds Ex. Ti. O 2 Note: All names will contain a roman numeral The Roman numeral will be the charge on each T-metal (see middle of PC) The charge will always be positive **The key is to determine the charge on the T-metals**.

Naming T-metal compounds Ex. Ti. O 2 Rules for naming T-metals 1) Write the formula for the compound Ti O 2 2) Write the charge of the non-metal Ti O 2 -2 3) Determine the charge on the T-metal… a) first determine the total negative charge O-2 = -4 total so +4 total needed to be neutral b) Determine what the charge of each metal must be to make it neutral. Ti. O 2 - this formula only has 1 Ti and 2 oxygens O-2 = -4 / Ti+? = 0 Ti+4

Naming T-metal compounds So far we have… +4 -2 Ti O 2 The roman numeral in the name of this compound is determined by the charge on the T-metal. Thus the name will be… Titanium IV oxide Remember, the + charge = the Roman numeral

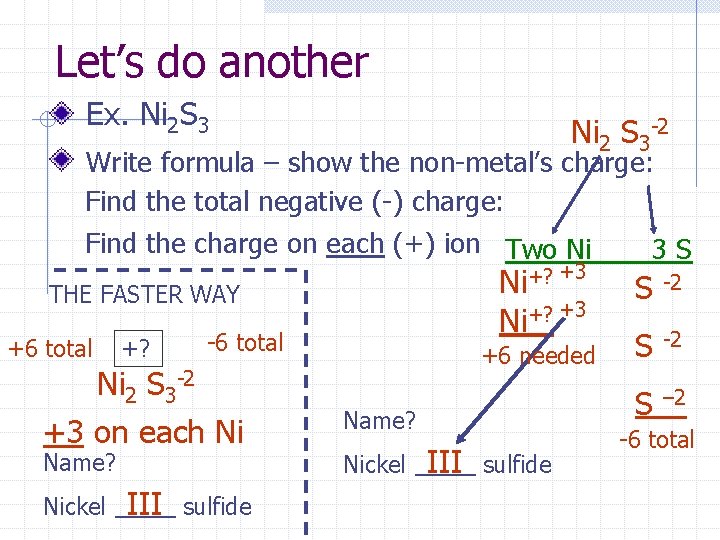
Let’s do another Ex. Ni 2 S 3 Ni 2 S 3 -2 Write formula – show the non-metal’s charge: Find the total negative (-) charge: Find the charge on each (+) ion Two Ni 3 S THE FASTER WAY +6 total +? Name? Nickel III sulfide S -2 +6 needed S -2 S – 2 Ni+? +3 -6 total Ni 2 S 3 -2 +3 on each Ni +3 +? Ni Name? Nickel III sulfide -6 total

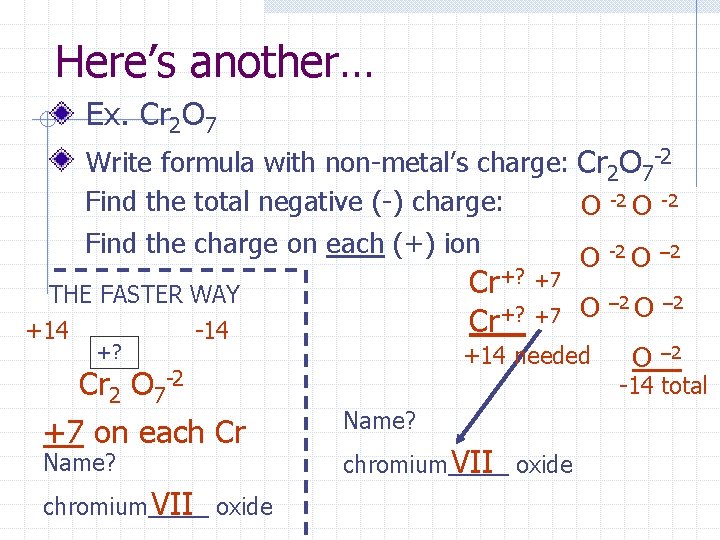
Here’s another… Ex. Cr 2 O 7 Write formula with non-metal’s charge: Cr 2 O 7 -2 Find the total negative (-) charge: O -2 Find the charge on each (+) ion O -2 O – 2 Cr+? THE FASTER WAY +14 -14 +? Cr 2 O 7 -2 +7 on each Cr Name? chromium VII oxide +7 +7 O +14 needed – 2 O – 2 -14 total Name? chromium VII oxide

Here’s another… Ex. Au 2 S Write formula with non-metal’s charge: Au 2 S-2 Find the total negative (-) charge: Find the charge on each (+) ion Au+? +1 THE FASTER WAY +2 -2 +? Au 2 S-2 +1 on each Au Name? gold I sulfide +2 needed Name? gold I sulfide S -2 -2 total

Here’s another… fast way only Ex. Fe 2 O 3 Write formula with non-metal’s charge: Fe 2 O 3 -2 Find the total negative (-) charge: Find the charge on each (+) ion +6 -6 +? Fe 2 O 3 -2 +3 on each Fe Name? iron III oxide

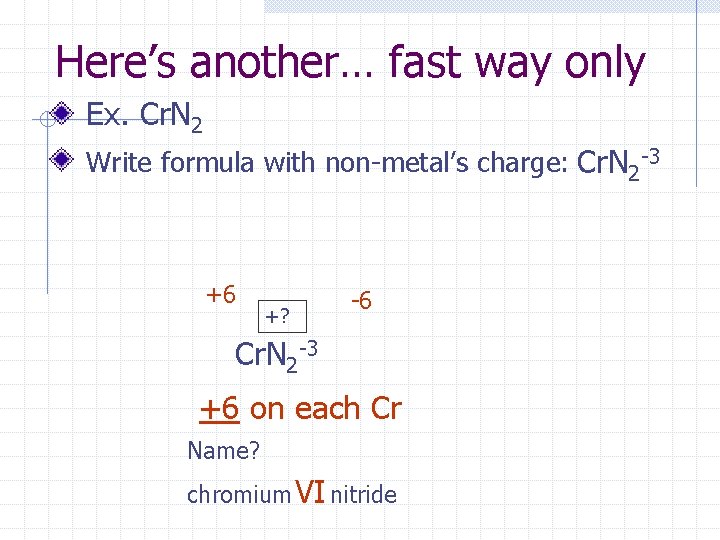
Here’s another… fast way only Ex. Cr. N 2 Write formula with non-metal’s charge: Cr. N 2 -3 +6 +? -6 Cr. N 2 -3 +6 on each Cr Name? chromium VI nitride

Here’s another… fast way only Ex. Fe 2 O 3 -2 Find the total negative (-) charge: Find the charge on each (+) ion +6 -6 +? Fe 2 O 3 -2 +3 on each Fe Name? iron III sulfide Jump to activity Writing NAMES

Writing names T-metals Fe. O Ni. Cl 2 Ti. O 2 Cu 2 O 3 Cr. F 2 Co. S 2

Writing names: mixed metals Al. Cl 3 Co. N Fe 2 O 3 Ca 3 P 2 Ni. O Cr 2 O 5

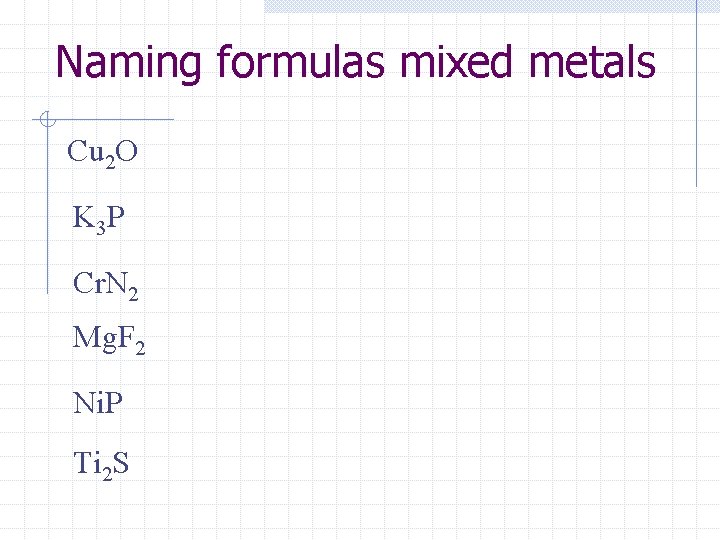
Naming formulas mixed metals Cu 2 O K 3 P Cr. N 2 Mg. F 2 Ni. P Ti 2 S

Activity last page: Naming formulas mixed-metals Ti. S 2 K 2 S Fe. N 3 Cr 2 O 7 Al 2 O 3

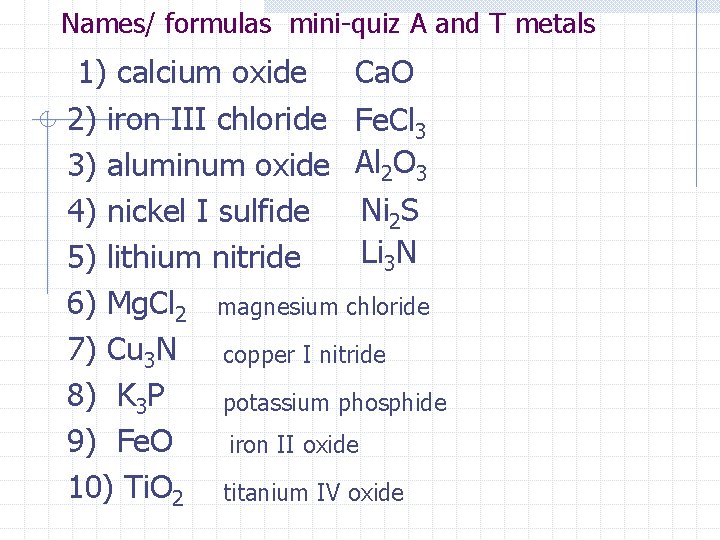
Names/ formulas mini-quiz A and T metals 1) calcium oxide Ca. O 2) iron III chloride Fe. Cl 3 3) aluminum oxide Al 2 O 3 Ni 2 S 4) nickel I sulfide Li 3 N 5) lithium nitride 6) Mg. Cl 2 magnesium chloride 7) Cu 3 N copper I nitride 8) K 3 P potassium phosphide 9) Fe. O iron II oxide 10) Ti. O 2 titanium IV oxide

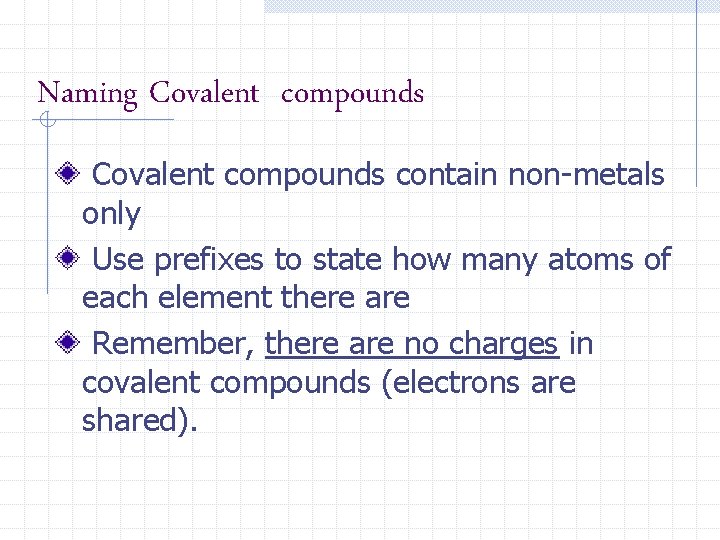
Naming Covalent compounds contain non-metals only Use prefixes to state how many atoms of each element there are Remember, there are no charges in covalent compounds (electrons are shared).

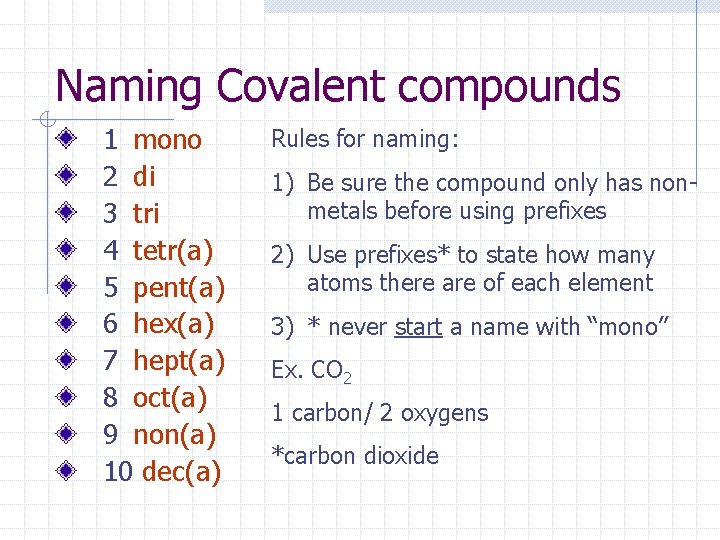
Naming Covalent compounds Here are the prefixes 1 mono 2 di 3 tri 4 tetr(a) 5 pent(a) 6 hex(a) 7 hept(a) 8 oct(a) 9 non(a) 10 dec(a)

Naming Covalent compounds 1 mono 2 di 3 tri 4 tetr(a) 5 pent(a) 6 hex(a) 7 hept(a) 8 oct(a) 9 non(a) 10 dec(a) Rules for naming: 1) Be sure the compound only has nonmetals before using prefixes 2) Use prefixes* to state how many atoms there are of each element 3) * never start a name with “mono” Ex. CO 2 1 carbon/ 2 oxygens *carbon dioxide

Naming Covalent compounds 1 mono 2 di 3 tri 4 tetr(a) 5 pent(a) 6 hex(a) 7 hept(a) 8 oct(a) 9 non(a) 10 dec(a) N 20 dinitrogen monoxide CO carbon monoxide OF 2 oxygen difluoride CF 4 carbon tetrafluoride

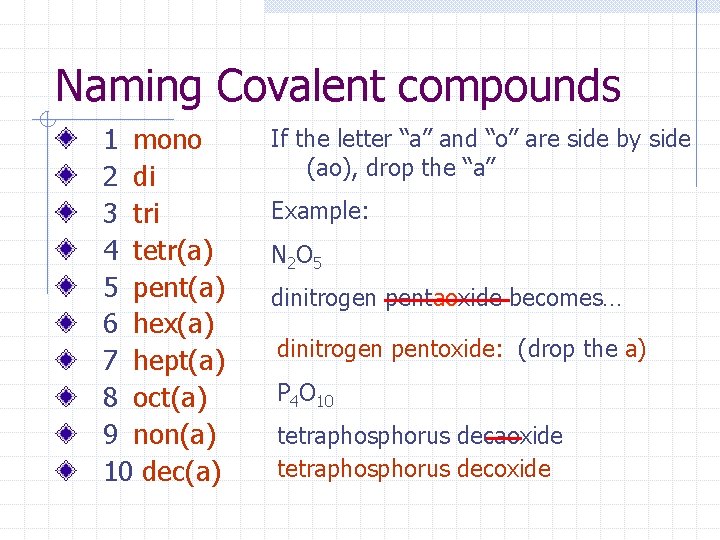
Naming Covalent compounds 1 mono 2 di 3 tri 4 tetr(a) 5 pent(a) 6 hex(a) 7 hept(a) 8 oct(a) 9 non(a) 10 dec(a) If the letter “a” and “o” are side by side (ao), drop the “a” Example: N 2 O 5 dinitrogen pentaoxide becomes… dinitrogen pentoxide: (drop the a) P 4 O 10 tetraphosphorus decaoxide tetraphosphorus decoxide

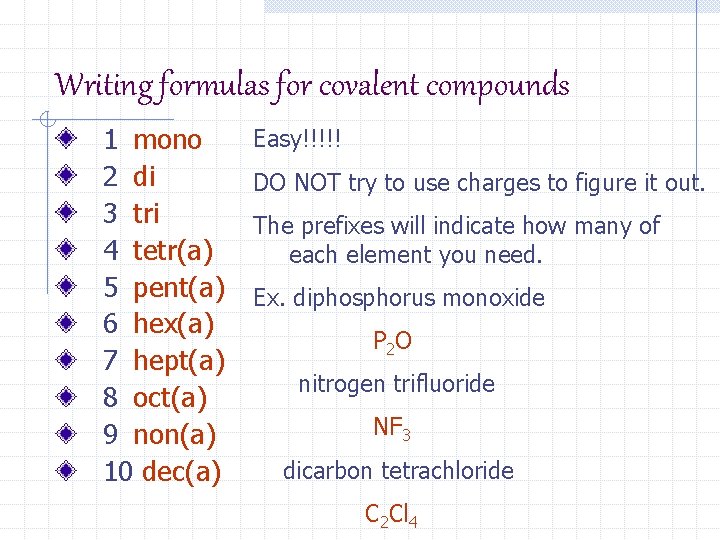
Writing formulas for covalent compounds 1 mono 2 di 3 tri 4 tetr(a) 5 pent(a) 6 hex(a) 7 hept(a) 8 oct(a) 9 non(a) 10 dec(a) Easy!!!!! DO NOT try to use charges to figure it out. The prefixes will indicate how many of each element you need. Ex. diphosphorus monoxide P 2 O nitrogen trifluoride NF 3 dicarbon tetrachloride C 2 Cl 4

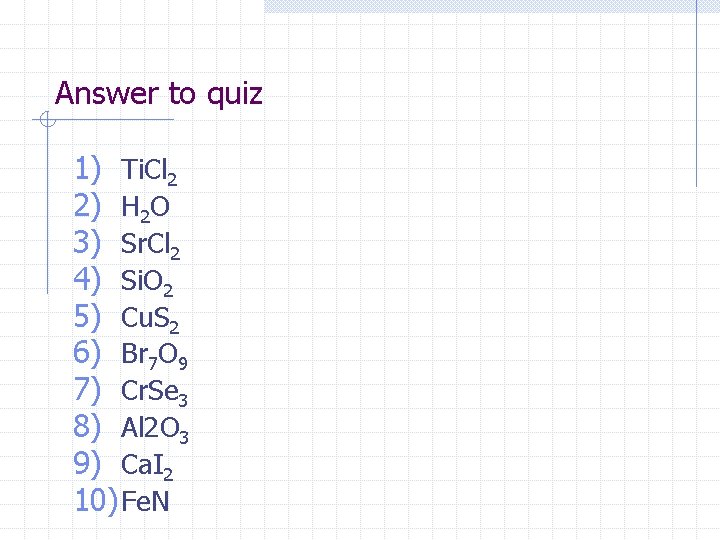
Answer to quiz 1) Ti. Cl 2 2) H 2 O 3) Sr. Cl 2 4) Si. O 2 5) Cu. S 2 6) Br 7 O 9 7) Cr. Se 3 8) Al 2 O 3 9) Ca. I 2 10) Fe. N

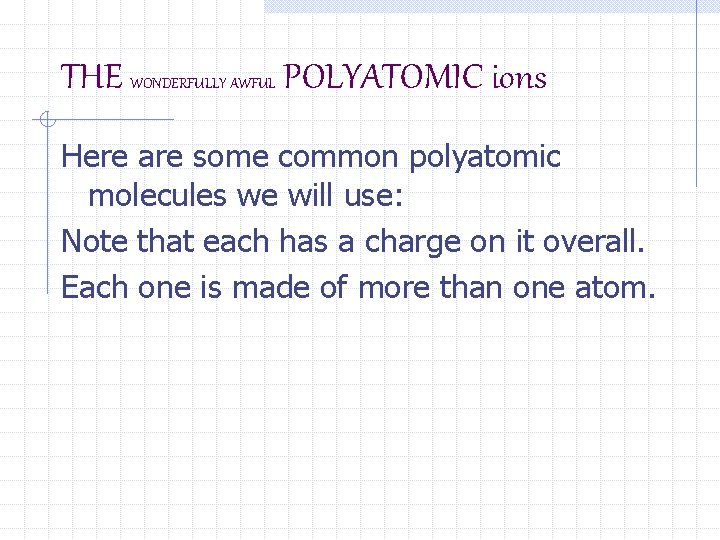
THE WONDERFULLY AWFUL POLYATOMIC ions Here are some common polyatomic molecules we will use: Note that each has a charge on it overall. Each one is made of more than one atom.

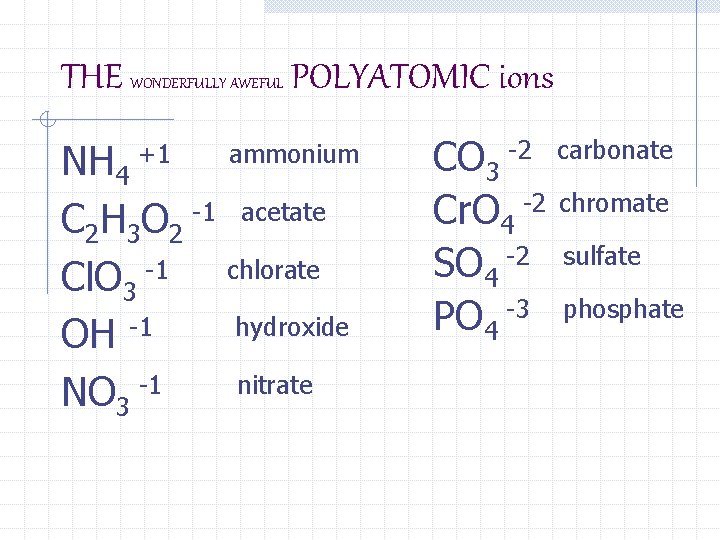
THE WONDERFULLY AWEFUL POLYATOMIC ions NH 4 C 2 H 3 O 2 -1 acetate Cl. O 3 -1 chlorate hydroxide OH -1 nitrate NO 3 -1 +1 ammonium CO 3 -2 carbonate Cr. O 4 -2 chromate SO 4 -2 sulfate PO 4 -3 phosphate

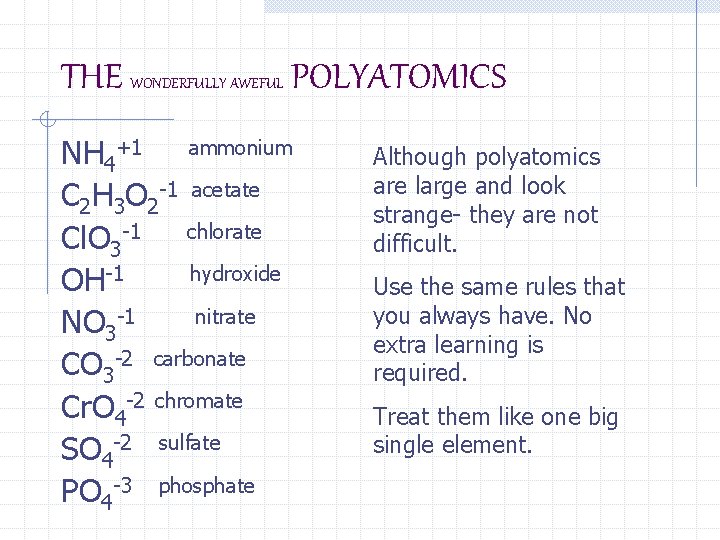
THE WONDERFULLY AWEFUL POLYATOMICS NH 4+1 ammonium C 2 H 3 O 2 -1 acetate Cl. O 3 -1 chlorate hydroxide OH-1 nitrate NO 3 -1 CO 3 -2 carbonate Cr. O 4 -2 chromate SO 4 -2 sulfate PO 4 -3 phosphate Although polyatomics are large and look strange- they are not difficult. Use the same rules that you always have. No extra learning is required. Treat them like one big single element.

Naming compounds with polyatomics… There are no new rules! If you have more than one polyatomic, simply use parentheses. Ex (Cl. O 3 -1)2: this is how to show two chlorates. Mg(Cl. O 3)2 - name this compound. Mg is an A-metal: use A-metal rules. Magnesium chlorate

Naming compounds with polyatomics… Cr(SO 4)2 name this compound. Cr is a T-metal: use T-metal rules. Chromium IV sulfate (NH 4)3 P Ammonium phosphide Ammonium is always +1 Treat it like an A metal Cu(NO 3)2 Copper II nitrate

Writing Formulas with polyatomics… No new rules! Just be sure it is neutral overall Ex. Potassium sulfate Potassium is an A-metal… K 2 SO 4 Calcium nitrate… Ca(NO 3)2

Writing Formulas with polyatomics… Titanium IV carbonate- write the formula Ti(CO 3)2 Iron VI phosphate Fe(PO 4)2 Gold I chromate Au 2 Cr. O 4 **Nickel II hydroxide Ni(OH)2

Practice mini quiz Name the following 1) Mg. Cl. O 3 2) Co 3(PO 4)2 3) K 2 SO 4 4) Cu(C 2 H 3 O 2)3 Write the formulas 5) Lithium phosphate 6) Iron II carbonate 7) Aluminum chromate 8) Titanium IV sulfate 9) Titanium IV sulfide

Practice mini quiz Name the following 1) Mg. Cl. O 3 2) Co 3(PO 4)2 3) K 2 SO 4 4) Cu(C 2 H 3 O 2)3 Answers 1) Magnesium chlorate 2) Cobalt II phosphate 3) Potassium sulfate 4) Copper III acetate

Practice mini quiz Write the formulas 5) Lithium phosphate 6) Iron II carbonate 7) Aluminum chromate 8) Titanium IV sulfate 9) Titanium IV sulfide answers 5) Li 3 PO 4 6) Fe. CO 3 7) Al 2(Cr. O 4)3 8) Ti(SO 4)2 9) Ti. S 2

Bell work: Date: Review for 3 min.

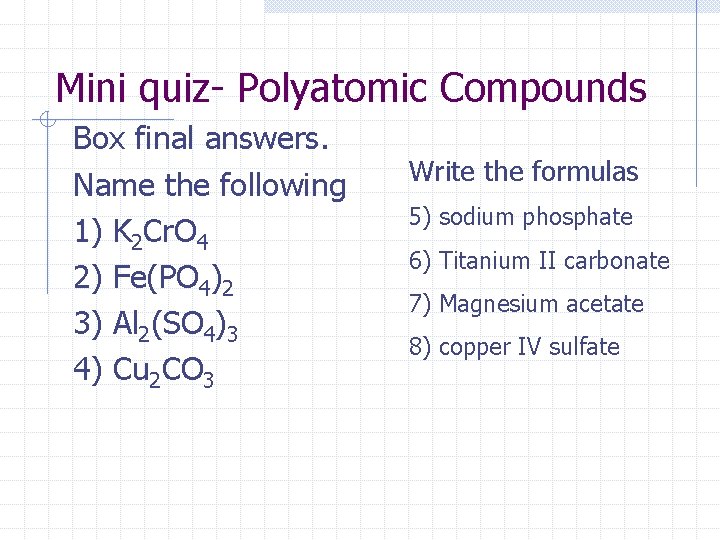
Mini quiz- Polyatomic Compounds Box final answers. Name the following 1) K 2 Cr. O 4 2) Fe(PO 4)2 3) Al 2(SO 4)3 4) Cu 2 CO 3 Write the formulas 5) sodium phosphate 6) Titanium II carbonate 7) Magnesium acetate 8) copper IV sulfate

Bell work: Date: Box final answer. Name the following [1) HF 2) HCl. O 3 3) HI 4) H 3 PO 4 Write the formulas 5) Hydroselenic acid 6) Nitric acid 7) Hydrosulfuric acid 8) Chromic acid

Practice Quiz: Date: 9/29/14 Name the following [1) K 2 Cr. O 4 2) Fe(PO 4)2 3) Al 2(SO 4)3 4) Cu 2 CO 3 5) H 3 PO 4 Write the formulas 6) sodium phosphate 7) Titanium II carbonate 8) Magnesium acetate 9) copper IV sulfate 10) Hydrosulfuric acid

Acids- mini quiz Box final answer. Name the following 1) H 2 Cr. O 4 2) HCl 3) H 3 PO 4 4) H 2 S Write the formulas 5) Hydroiodic acid 6) Nitric acid 7) Hydrosulfuric acid 8) Carbonic acid

Acids- mini quiz II Name the following 1) H 2 S 2) H 2 CO 3 3) HNO 3 4) HBr 5) H 3 PO 4 Write the formulas 6) Hydroiodic acid 7) acetic acid 8) phosphoric acid 9) hydrochloric acid 10) Nitric acid

All Compounds- warm up! Name the following 1) Ca. Cl 2 2) H 2 CO 3 3) Fe. S 2 4) Br 2 P 3 Write the formulas 5) sulfuric acid 6) nickel I oxide 7) carbon tetrafluoride 8) aluminum oxide

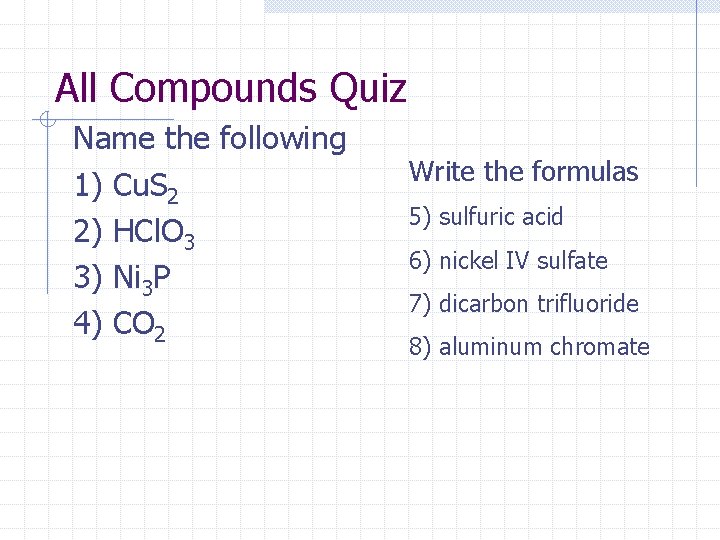
All Compounds Quiz Name the following 1) Cu. S 2 2) HCl. O 3 3) Ni 3 P 4) CO 2 Write the formulas 5) sulfuric acid 6) nickel IV sulfate 7) dicarbon trifluoride 8) aluminum chromate