Rules for Naming Compounds and Writing Formulas Naming



- Slides: 8

Rules for Naming Compounds and Writing Formulas

Naming Binary Ionic Compounds • Determine whether the element would lose or gain electrons when bonding to get a full outer energy level and determine the charge the ion would have resulting from losing or gaining electrons. (practice problems Ch 6 #2, 16 -17) • Positive ions are called cations and negative ions are called anions. (practice problems #18 -19) • Cations names are taken straight from the periodic table. For Anions names, the end of the name on the periodic table is replaced with “ide”. (practice problems #1, 8 -9, 2223) • When cations and anions come together to make a compound, the cation is always written first and capitalized and the anion is written second (practice problems #26 -27)

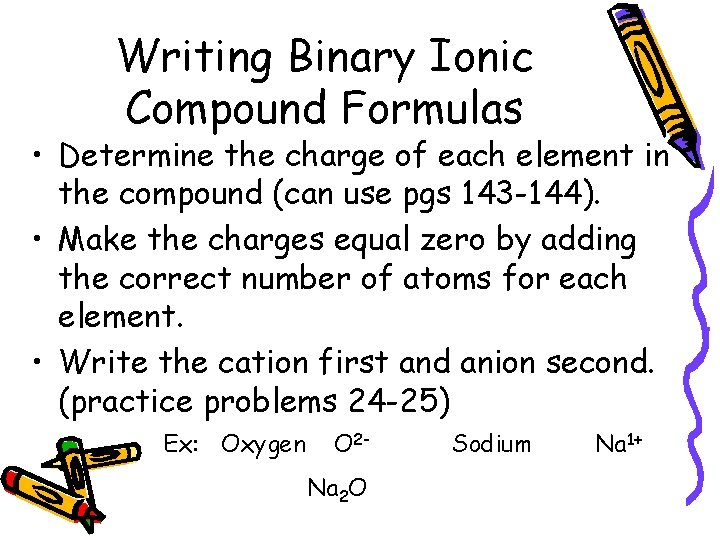
Writing Binary Ionic Compound Formulas • Determine the charge of each element in the compound (can use pgs 143 -144). • Make the charges equal zero by adding the correct number of atoms for each element. • Write the cation first and anion second. (practice problems 24 -25) Ex: Oxygen O 2 Na 2 O Sodium Na 1+

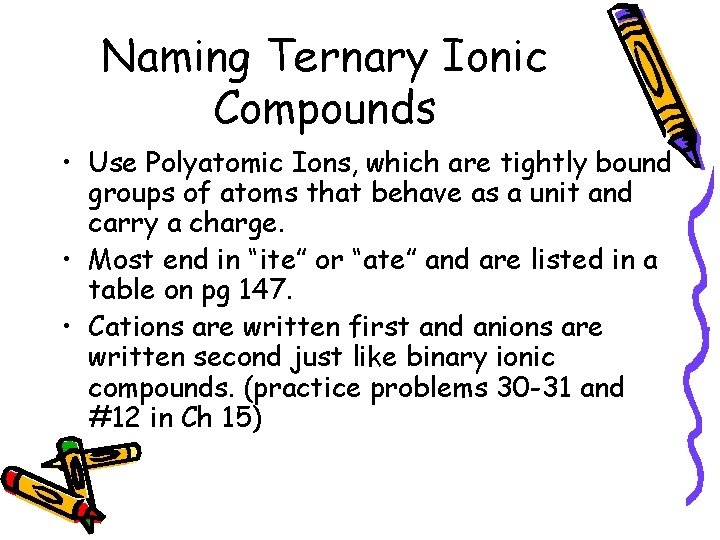
Naming Ternary Ionic Compounds • Use Polyatomic Ions, which are tightly bound groups of atoms that behave as a unit and carry a charge. • Most end in “ite” or “ate” and are listed in a table on pg 147. • Cations are written first and anions are written second just like binary ionic compounds. (practice problems 30 -31 and #12 in Ch 15)

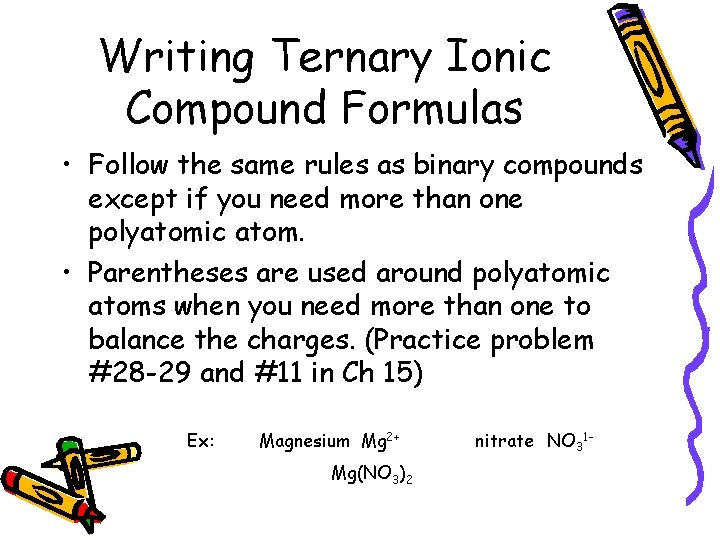
Writing Ternary Ionic Compound Formulas • Follow the same rules as binary compounds except if you need more than one polyatomic atom. • Parentheses are used around polyatomic atoms when you need more than one to balance the charges. (Practice problem #28 -29 and #11 in Ch 15) Ex: Magnesium Mg 2+ Mg(NO 3)2 nitrate NO 31 -

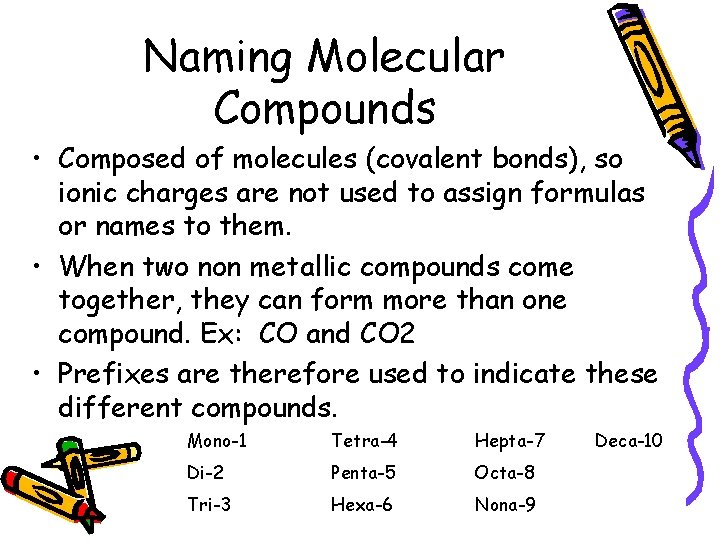
Naming Molecular Compounds • Composed of molecules (covalent bonds), so ionic charges are not used to assign formulas or names to them. • When two non metallic compounds come together, they can form more than one compound. Ex: CO and CO 2 • Prefixes are therefore used to indicate these different compounds. Mono-1 Tetra-4 Hepta-7 Di-2 Penta-5 Octa-8 Tri-3 Hexa-6 Nona-9 Deca-10

Naming Acids • Acids are compounds that produce hydrogen ions when dissolved in water. • Acids are an anion connected to a hydrogen ion. • If it a monatomic anion, then you add “hydro” on the front, end it with “ic” and then add acid. Ex: HCl Hydrochloric acid • If it is a polyatomic anion that end with “ate”, change ending to “ic” and add acid. Ex: H 2 SO 4 Sulfuric acid. • If it is a polyatomic anion that ends with “ite”, change ending to “ous” and add acid. Ex: HNO 2 Nitrous acid

• Use flow charts on pg 161 and 162 to help you with naming and writing formulas