Naming Ionic Compounds and Formulas Ionic Compounds Chemical















- Slides: 23

Naming Ionic Compounds and Formulas

Ionic Compounds Chemical Name: The chemical name tells you what ions (charged atoms) are in the compound eg. Sodium chloride: has sodium and chlorine in it Calcium oxide:

Ionic Compounds Chemical Name: The chemical name tells you what ions (charged atoms) are in the compound eg. Sodium chloride: has sodium and chlorine in it Calcium oxide: has calcium and oxygen in it

How do we write chemical names? • Rules for naming compounds 1) Start the name with the positive ion (metal)

How do we write chemical names? • Rules for naming compounds 1) Start the name with the positive ion (metal) 2) The second ion is the negative ion (non-metal)

How do we write chemical names? • Rules for naming compounds 1) Start the name with the positive ion (metal) 2) The second ion is the negative ion (non-metal) 3) The name of the metal stays the same

How do we write chemical names? • Rules for naming compounds 1) Start the name with the positive ion (metal) 2) The second ion is the negative ion (non-metal) 3) The name of the metal stays the same 4) The name of the non-metal had a new ending (the “ide” ending)

What would be the name of the following compounds Chlorine and Magnesium: ____________ Nitrogen and Iron: _______________ Calcium and Bromide: ______________ Silver and Fluorine: _______________

What would be the name of the following compounds Chlorine and Magnesium: Magnesium chloride Nitrogen and Iron: Iron nitride Calcium and Bromide: Calcium bromide Silver and Fluorine: Silver fluoride


Write the names of the following compounds 1) Al. I 3 2) Na 2 O 3) Mg 3 P 2 4) Ag. I 5) K 2 S 6) Rb. F 7) Ag 3 N 8) Ca. I 2 Aluminum iodide Sodium oxide Magnesium phosphide Silver iodide

Write the names of the following compounds 1) Al. I 3 2) Na 2 O 3) Mg 3 P 2 4) Ag. I 5) K 2 S 6) Rb. F 7) Ag 3 N 8) Ca. I 2 Aluminum iodide Sodium oxide Magnesium phosphide Silver iodide Potassium sulphide Rubidium fluoride Silver nitride Calcium iodide

Ionic Compounds Chemical Formula: The chemical formula tells you what ions (charged atoms) are in the compound and the relative number of the ions.

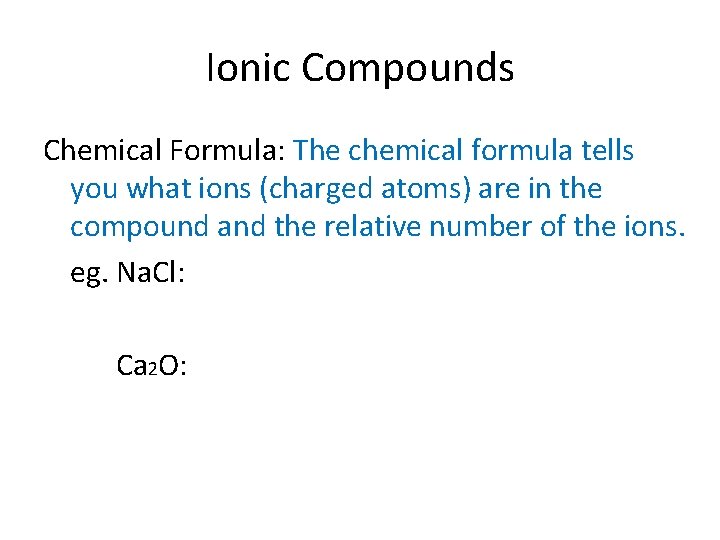
Ionic Compounds Chemical Formula: The chemical formula tells you what ions (charged atoms) are in the compound and the relative number of the ions. eg. Na. Cl: Ca 2 O:

Ionic Compounds Chemical Formula: The chemical formula tells you what ions (charged atoms) are in the compound and the relative number of the ions. eg. Na. Cl: has sodium and chlorine in it and it has 1 sodium ion to 1 chlorine ion Ca 2 O: has calcium and oxygen in it and there is 2 calcium ions for every 1 oxygen ion

Writing Chemical Formulas • Steps for writing a chemical formula 1) Write down the ions with charges 2) Determine how you are going to balance the charges

Writing Chemical Formulas • Steps for writing a chemical formula 1) Write down the ions with charges 2) Determine how you are going to balance the charges 3) Note the ratio of positive to negative ions 4) Write the subscript into the formula

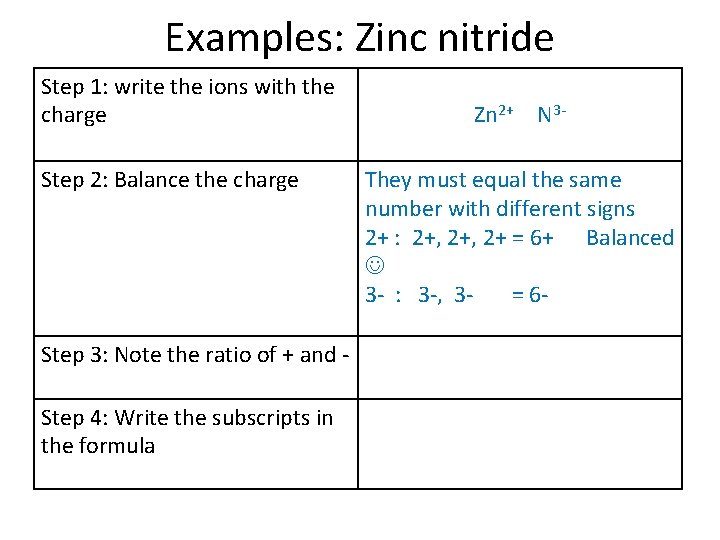
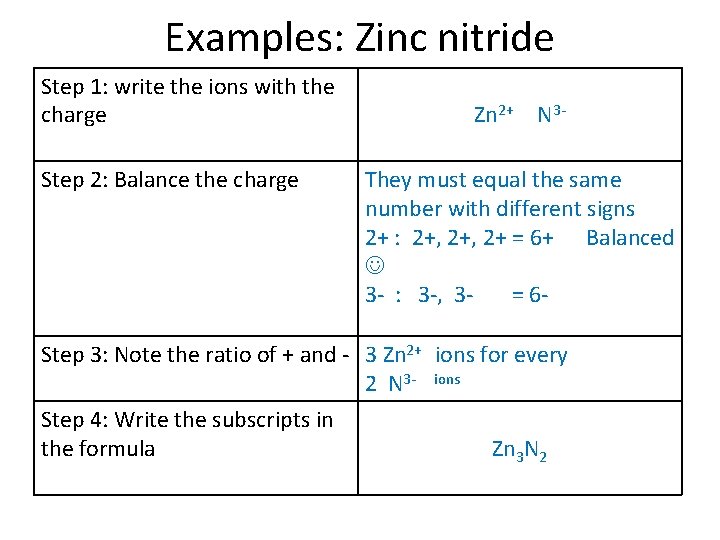
Examples: Zinc nitride Step 1: write the ions with the charge Step 2: Balance the charge Step 3: Note the ratio of + and Step 4: Write the subscripts in the formula Zn 2+ N 3 -

Examples: Zinc nitride Step 1: write the ions with the charge Step 2: Balance the charge Step 3: Note the ratio of + and Step 4: Write the subscripts in the formula Zn 2+ N 3 They must equal the same number with different signs 2+ : 2+, 2+ = 6+ Balanced 3 - : 3 -, 3= 6 -

Examples: Zinc nitride Step 1: write the ions with the charge Step 2: Balance the charge Zn 2+ N 3 They must equal the same number with different signs 2+ : 2+, 2+ = 6+ Balanced 3 - : 3 -, 3= 6 - Step 3: Note the ratio of + and - 3 Zn 2+ ions for every 2 N 3 - ions Step 4: Write the subscripts in the formula Zn 3 N 2

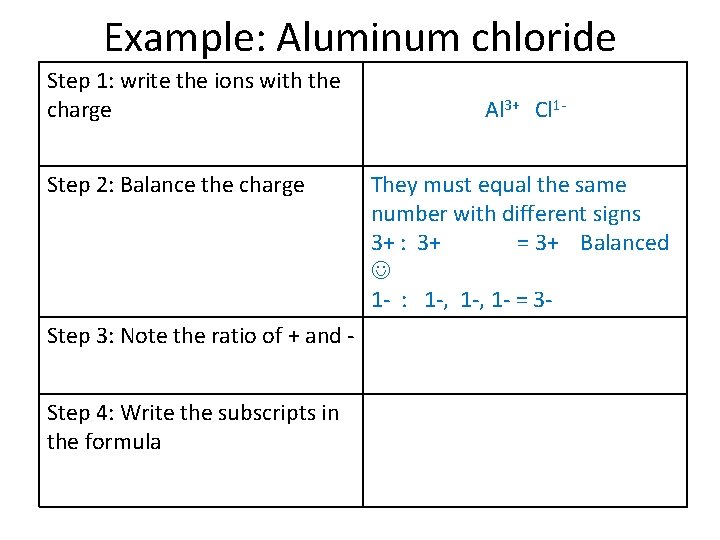
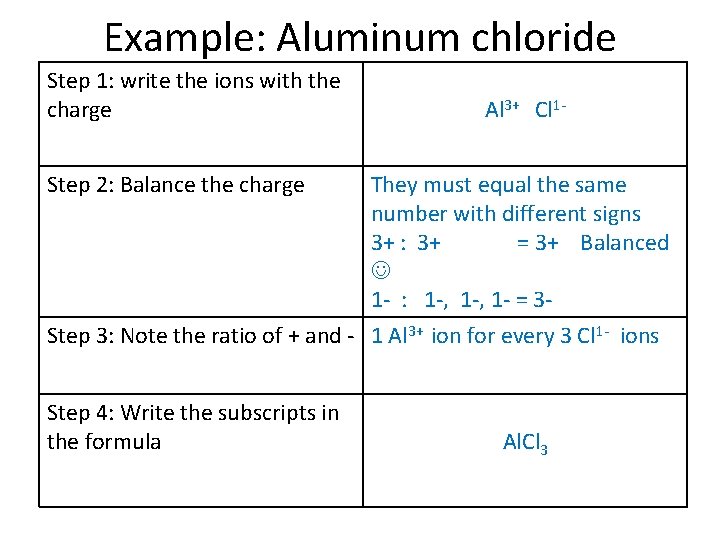
Example: Aluminum chloride Step 1: write the ions with the charge Step 2: Balance the charge Step 3: Note the ratio of + and Step 4: Write the subscripts in the formula Al 3+ Cl 1 They must equal the same number with different signs 3+ : 3+ = 3+ Balanced 1 - : 1 -, 1 - = 3 -

Example: Aluminum chloride Step 1: write the ions with the charge Al 3+ Cl 1 - Step 2: Balance the charge They must equal the same number with different signs 3+ : 3+ = 3+ Balanced 1 - : 1 -, 1 - = 3 Step 3: Note the ratio of + and - 1 Al 3+ ion for every 3 Cl 1 - ions Step 4: Write the subscripts in the formula Al. Cl 3

Examples Step 1: write the ions with the charge Step 2: Balance the charge Step 3: Note the ratio of + and Step 4: Write the subscripts in the formula