Naming Compounds Covalent naming Chemical Formulas Naming Molecules







- Slides: 11

Naming Compounds Covalent naming

Chemical Formulas Naming Molecules • Recently, we have worked with naming and writing formulas for ionicly bonded salts • Now we will do similar work with covalently bonded compounds (molecules)

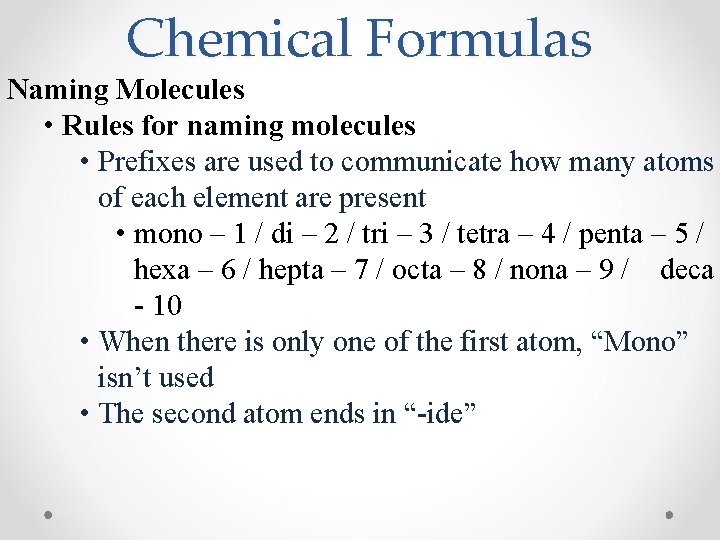
Chemical Formulas Naming Molecules • Rules for naming molecules • Prefixes are used to communicate how many atoms of each element are present • mono – 1 / di – 2 / tri – 3 / tetra – 4 / penta – 5 / hexa – 6 / hepta – 7 / octa – 8 / nona – 9 / deca - 10 • When there is only one of the first atom, “Mono” isn’t used • The second atom ends in “-ide”

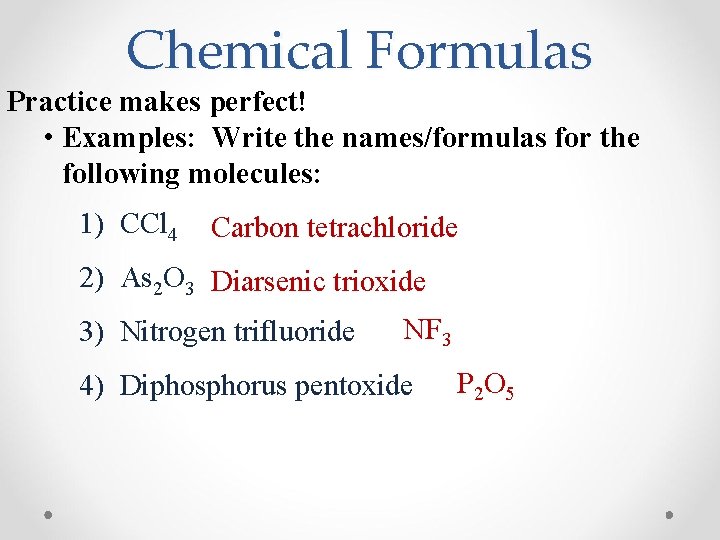
Chemical Formulas Practice makes perfect! • Examples: Write the names/formulas for the following molecules: 1) CCl 4 Carbon tetrachloride 2) As 2 O 3 Diarsenic trioxide 3) Nitrogen trifluoride NF 3 4) Diphosphorus pentoxide P 2 O 5

Chemical Formulas Practice makes perfect! • Write the names/formulas for the following molecules: 1) SF 6 sulfur hexafluoride 2) N 2 O dinitrogen oxide 3) NO 2 nitrogen dioxide 4) dichlorine octoxide Cl 2 O 8 5) dinitrogen tetroxide N 2 O 4

Naming Compounds Acid/Base naming

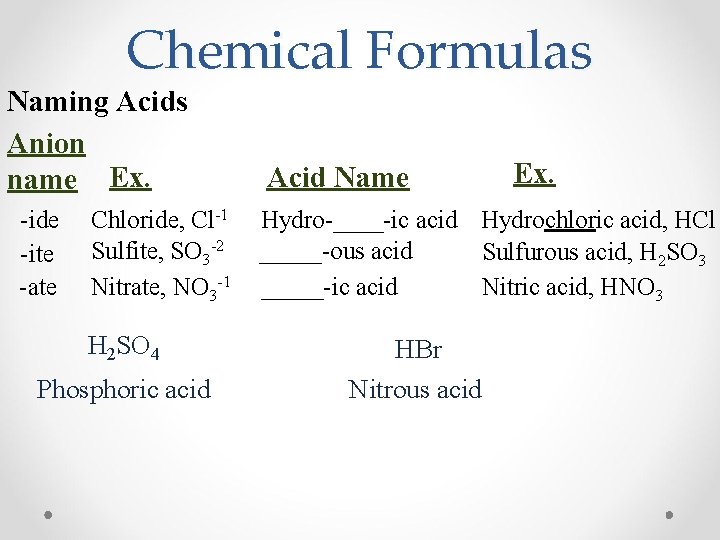
Chemical Formulas Naming Acids • Acids: compounds which release 1 or more H+ ions in water • When we name them, we consider them to be a hydrogen cation bonded to an anion • The name of the acid depends on the name of the anion

Chemical Formulas Naming Acids Anion name Ex. -ide -ite -ate Chloride, Cl-1 Sulfite, SO 3 -2 Nitrate, NO 3 -1 H 2 SO 4 Phosphoric acid Acid Name Ex. Hydro-____-ic acid Hydrochloric acid, HCl _____-ous acid Sulfurous acid, H 2 SO 3 _____-ic acid Nitric acid, HNO 3 HBr Nitrous acid

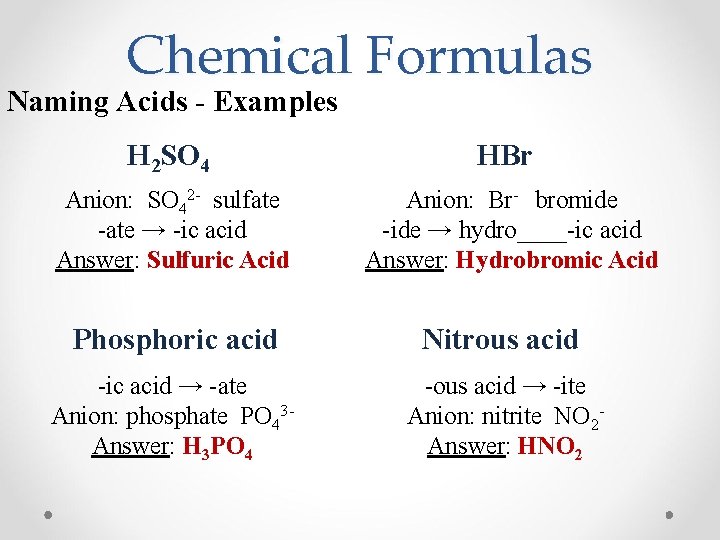
Chemical Formulas Naming Acids - Examples H 2 SO 4 HBr Anion: SO 42 - sulfate -ate → -ic acid Answer: Sulfuric Acid Anion: Br- bromide -ide → hydro____-ic acid Answer: Hydrobromic Acid Phosphoric acid Nitrous acid -ic acid → -ate Anion: phosphate PO 43 Answer: H 3 PO 4 -ous acid → -ite Anion: nitrite NO 2 Answer: HNO 2

Chemical Formulas Naming Bases • Bases: compounds which release 1 or more OH- ions in water • Just name them as the hydroxide ionic compounds that they are! • ex) Na. OH sodium hydroxide • ex) Ca(OH)2 calcium hydroxide • When writing base formulas, be careful of the charge of the cation!

Naming Compounds La fin!