Topic Naming Ionic Compounds Do Now Write formulas









- Slides: 14

Topic: Naming Ionic Compounds Do Now: Write formulas for the following 1. Na and Cl 2. Mg and I 3. Al and S Na+1 Cl-1 = Na. Cl Mg+2 I-1 = Mg. I 2 Al+3 S-2 = Al 2 S 3

Naming Binary Ionic Compounds there is a flow chart for you to use add to your reference table packet 1. Always name metal (the cation/+ ion) first 2. Leave a space 3. Write stem of nonmetal (the anion/ - ion) and Add ending “ide” to nonmetal Binary = only 2 types of elements (two capital letters)

Stems of nonmetals Nitr Ox Fluor Phosph Sulf Chlor Arsen Selen Brom Hydr is the stem for H Tellur Iod

Metals with one oxidation state • • Ca. O Calcium Oxide Ba. S Barium Sulfide Al. N Aluminum Nitride Li. Cl Lithium Chloride Al 2 Se 3 Aluminum Selenide Na 2 O Sodium Oxide K 3 N Potassium Nitride Mg. F 2 Magnesium Fluoride

The First Step in Naming • Find metal on PT • If metal has only one oxidation state it’s easy • If metal has more than one oxidation state, there’s an extra step

Metals with more than 1 oxidation state • Use formula to figure out which oxidation state metal ion has • Ex: Fe can be Fe+2 or Fe+3 » Fe. O vs Fe 2 O 3 – two different compounds so cannot both be called iron oxide – so we have to use Roman Numeral to Tell which Fe (Fe+2 or Fe+3) we are using – Iron (II) Oxide –uses Fe+2 – Irons (III) Oxide – use Fe+3

When deterring Oxidation State of Metal being used, assigned the nonmetal it’s oxidation number first (will be the top oxidation number from the PT)

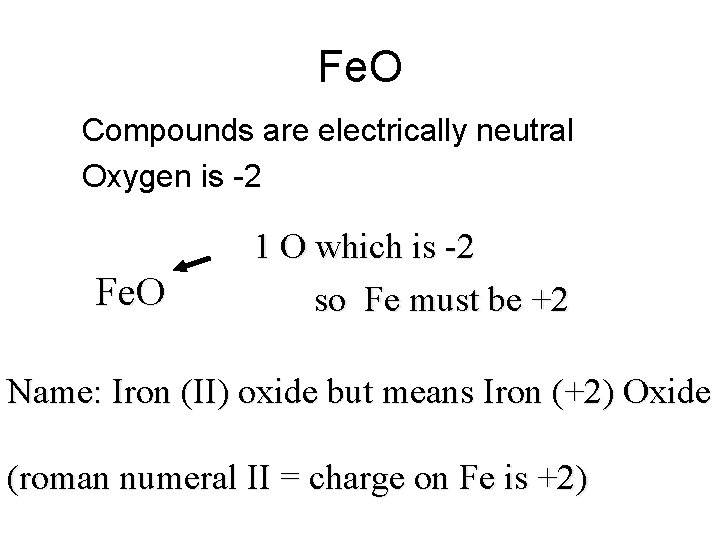
Fe. O • Compounds are electrically neutral • Oxygen is -2 Fe. O 1 O which is -2 so Fe must be +2 Name: Iron (II) oxide but means Iron (+2) Oxide (roman numeral II = charge on Fe is +2)

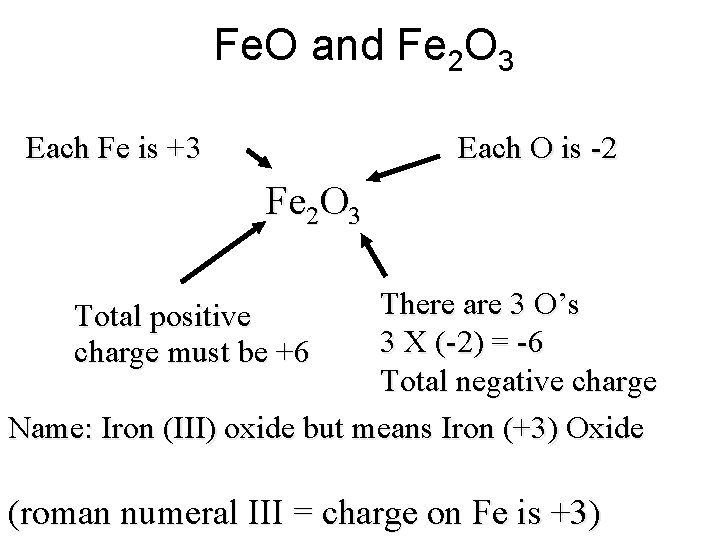
Fe. O and Fe 2 O 3 Each O is -2 Each Fe is +3 Fe 2 O 3 There are 3 O’s 3 X (-2) = -6 Total negative charge Name: Iron (III) oxide but means Iron (+3) Oxide Total positive charge must be +6 (roman numeral III = charge on Fe is +3)

Name the following • • Ti. Cl 3 Titanium (III) chloride Mn. O 2 Manganese (IV) oxide Co 2 O 3 Cobalt (III) oxide Pd. Br 2 Palladium (II) bromide Au. Cl 3 Gold (III) chloride Mo. N Molybdenum (III) nitride Mn. O Manganese (II) oxide Ti. O Titanium (II) oxide

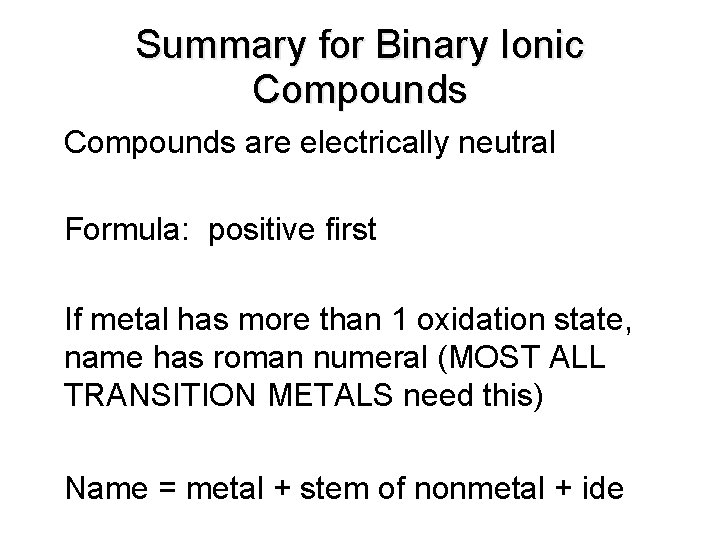
Summary for Binary Ionic Compounds are electrically neutral • Formula: positive first • If metal has more than 1 oxidation state, name has roman numeral (MOST ALL TRANSITION METALS need this) • Name = metal + stem of nonmetal + ide

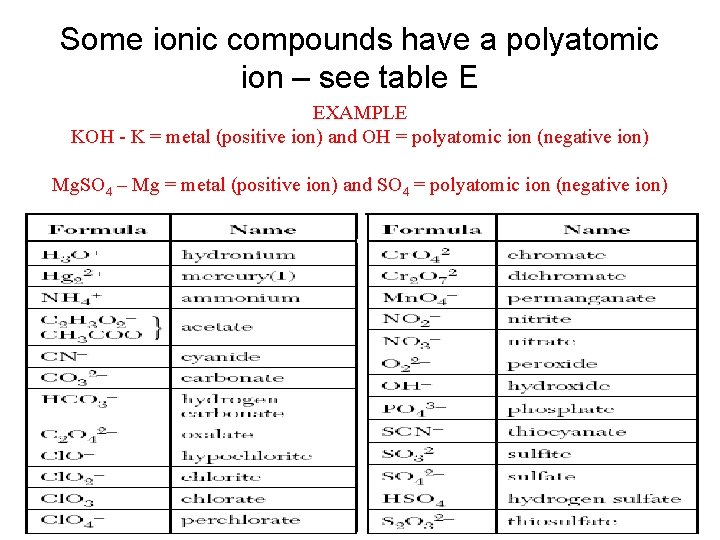
Some ionic compounds have a polyatomic ion – see table E EXAMPLE KOH - K = metal (positive ion) and OH = polyatomic ion (negative ion) Mg. SO 4 – Mg = metal (positive ion) and SO 4 = polyatomic ion (negative ion)

Naming compounds with polyatomics • • polyatomic ions have names (Table E) naming is parallel to binary naming positive always written first if (+)’ve ion is a metal, check to see how many oxidation states it has – if > 1 then name must have roman numeral • if (–)’ve is polyatomic - 2 nd part of name is name of polyatomic (don’t modify ending)

Name the following • • Na. OH Sodium hydroxide KHCO 3 Potassium hydrogen carbonate Li. NO 3 Lithium nitrate Ca. SO 4 Calcium sulfate Al(NO 3)3 Aluminum nitrate Fe(OH)2 Iron (II) hydroxide Cu. SO 4 Copper (II) sulfate Cu. SCN Copper (I) thiocyanate