Chapter 9 Naming Compounds Writing Formulas Systematic Naming
































































- Slides: 82

Chapter 9 Naming Compounds Writing Formulas

Systematic Naming There are too many compounds to remember the names of them all. l Compound is made of two or more elements. l Put together atoms. l Name should tell us how many and what type of atoms. l

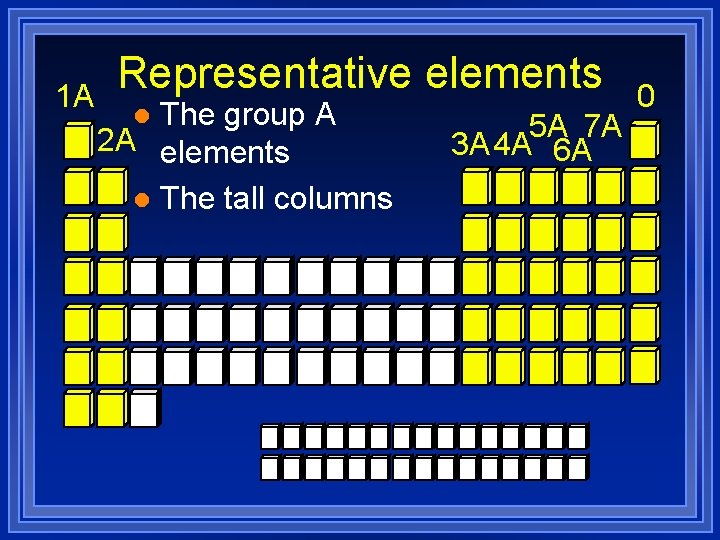
Periodic Table More than a list of elements. l Put in columns because of similar properties. l Each column is called a group. l

1 A Representative elements The group A 2 A elements l The tall columns l 5 A 7 A 3 A 4 A 6 A 0

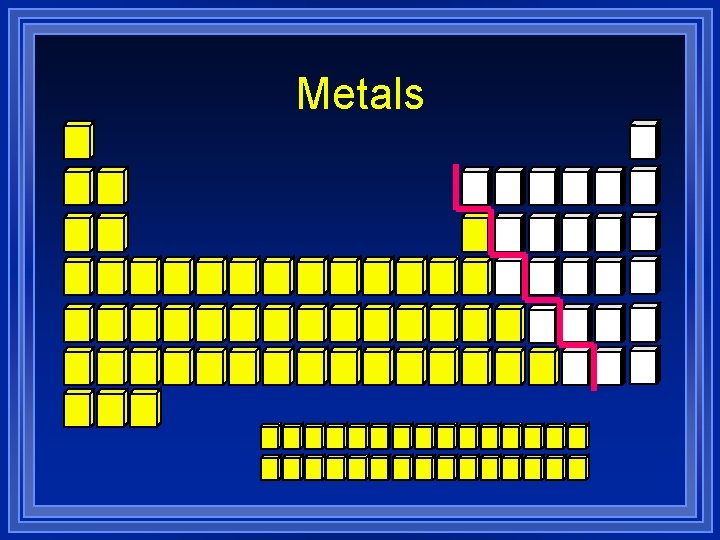
Metals

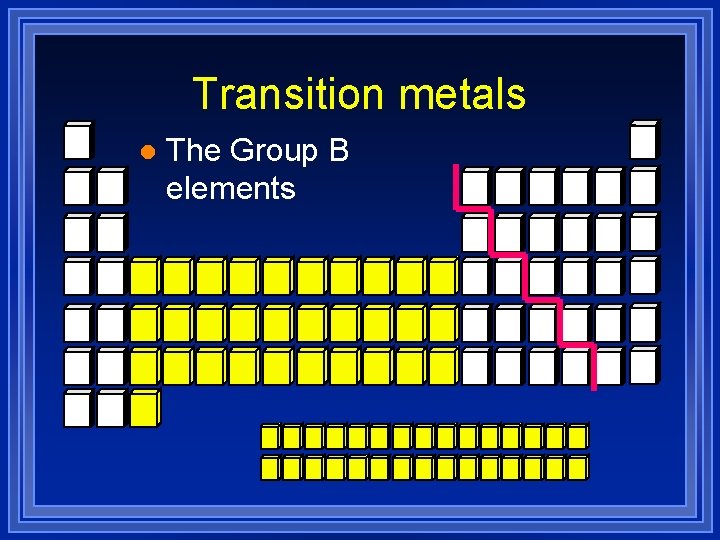
Transition metals l The Group B elements

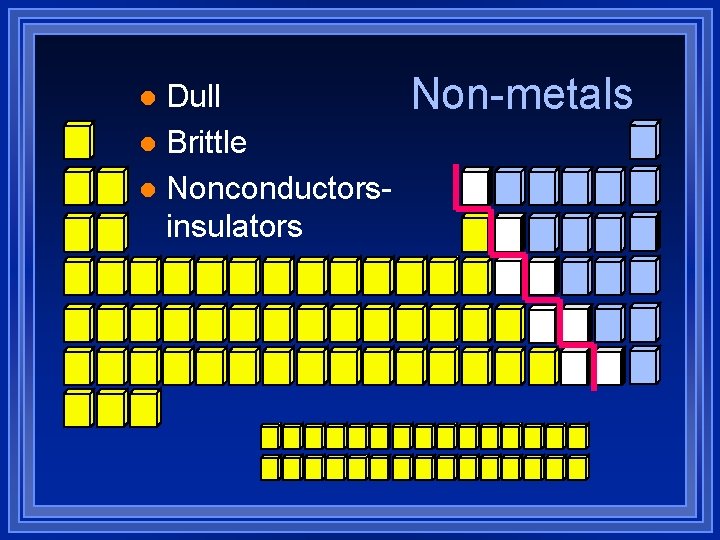
Dull l Brittle l Nonconductorsinsulators l Non-metals

Metalloids or Semimetals Properties of both l Semiconductors l

Atoms and ions Atoms are electrically neutral. l Same number of protons and electrons. l Ions are atoms, or groups of atoms, with a charge. l Different numbers of protons and electrons. l Only electrons can move. l Gain or lose electrons. l

Anion A negative ion. l Has gained electrons. l Non metals can gain electrons. l Charge is written as a superscript on the right. l 1 F 2 O Has gained one electron Has gained two electrons

Cations Positive ions. l Formed by losing electrons. l More protons than electrons. l Metals form cations. l 1+ K Has lost one electron 2+ Ca Has lost two electrons

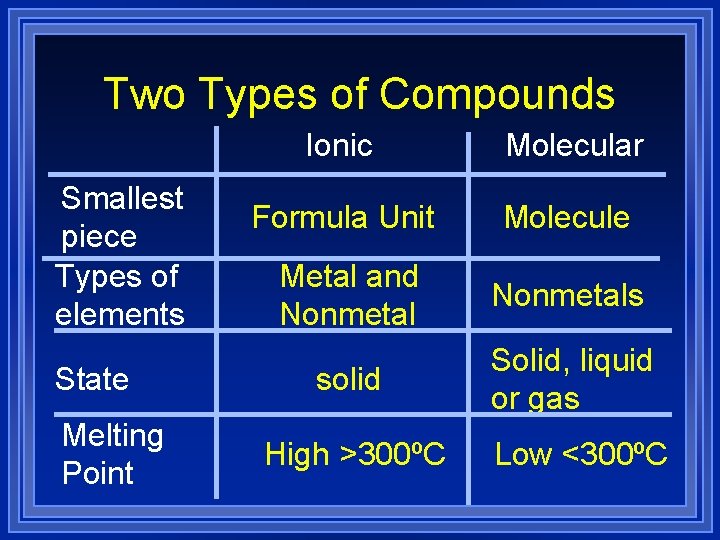
Two Types of Compounds 1 Molecular compounds Made of molecules. l Made by joining nonmetal atoms together into molecules. l

Two Types of Compounds 2 Ionic Compounds Made of cations and anions. l Metals and nonmetals. l The electrons lost by the cation are gained by the anion. l The cation and anions surround each other. l Smallest piece is a FORMULA UNIT. l

Two Types of Compounds Smallest piece Types of elements State Melting Point Ionic Molecular Formula Unit Molecule Metal and Nonmetals solid Solid, liquid or gas High >300ºC Low <300ºC

Chemical Formulas Shows the kind and number of atoms in the smallest piece of a substance. l Molecular formula- number and kinds of atoms in a molecule. l CO 2 l C 6 H 12 O 6 l

Formula Unit l The smallest whole number ratio of atoms in an ionic compound. l Ions surround each other so you can’t say which is hooked to which.

Charges on ions l For most of Group A elements, location on the Periodic Table can tell what kind of ion they form l Elements in the same group have similar properties. l Including the charge when they are ions.

Charge in groups 1 A, 2 A and 3 A is the group number 1+ 2+ in 5 A, 6 A and 7 A is 3+ 3 - 2 - 1 the group number 8

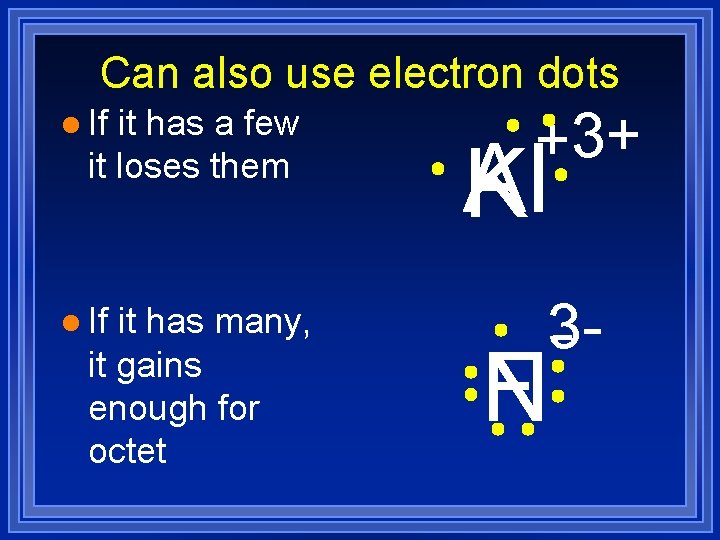
Can also use electron dots l If it has a few it loses them l If it has many, it gains enough for octet +3+ Al K 3 - F N

What about the others? l We have to figure those out some other way. l More on this later.

Naming ions l Cation- if the charge is always the same (Group A) just write the name of the metal. l Most transition metals can have more than one type of charge. l Indicate the charge with Roman numerals in parenthesis. l Co 2+ Cobalt(II) ion

Naming ions l. A few, like silver, zinc and cadmium only form one kind of ion l Don’t get roman numerals l Ag+ silver ion l Zn 2+ zinc ion l Cd 2+ cadmium ion

l. Na 1+ 2+ l. Ca 3+ l. Al l. Fe 3+ l. Fe 2+ 2+ l. Pb 1+ l. Li Name these Sodium ion Calcium ion Aluminum ion Iron(III) ion Iron(II) ion Lead(II) ion Lithium ion

Write Formulas for these l. Potassium ion l. Magnesium l. Copper(II) ion K 1+ 2+ Mg 2+ Cu 6+ Cr l. Chromium(VI) ion 2+ Ba l. Barium ion l. Mercury(II) ion 2+ Hg

Naming Anions l Anions are always the same. l Change the element ending to – ide 1 l F Fluorine

Naming Anions l Anions are always the same. l Change the element ending to – ide 1 l F Fluorin

Naming Anions l Anions are always the same l Change the element ending to – ide 1 l F Fluori

Naming Anions l Anions are always the same l Change the element ending to – ide 1 l F Fluor

Naming Anions l Anions are always the same l Change the element ending to – ide 1 l F Fluori

Naming Anions l Anions are always the same l Change the element ending to – ide 1 l F Fluorid

Naming Anions l Anions are always the same l Change the element ending to – ide 1 l F Fluoride

Naming Anions l Anions are always the same l Change the element ending to – ide 1 l F Fluoride ion

Name these l. Cl 1 l. N 3 l. Br 1 l. O 2 l. Ga 3+ Chloride ion Nitride ion Bromide ion Oxide ion Gallium ion

Write these l. Sulfide ion S 2 l. Iodide ion I 1 l. Phosphide ion P 3 l. Strontium ion Sr 2+

Polyatomic ions l Groups of atoms that stay together and have a charge. l Covalently bonded l You must memorize these. (pg 257)

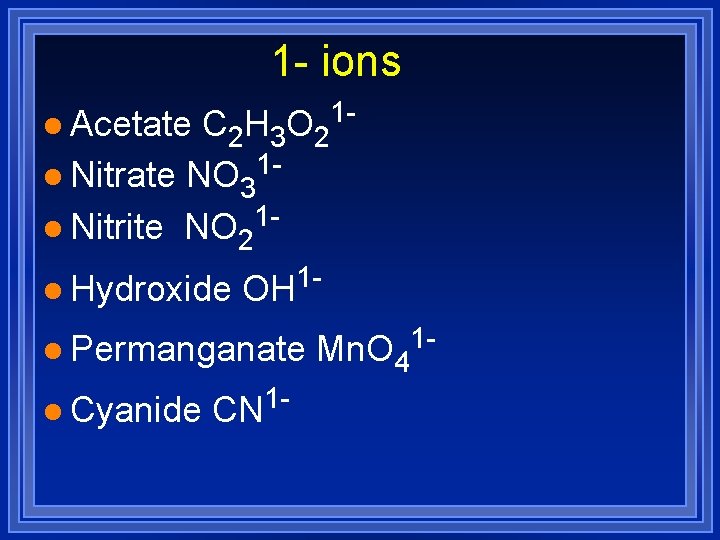
1 - ions C 2 H 3 O 21 l Nitrate NO 31 l Nitrite NO 21 l Acetate l Hydroxide 1 OH l Permanganate l Cyanide CN 1 - Mn. O 4 1 -

1 - ions Cl. O 41 l Chlorate Cl. O 31 l Chlorite Cl. O 21 l Perchlorate 1 l Hypochlorite

2 - ions l Sulfate 2 - SO 4 2 l Sulfite SO 3 2 l Carbonate CO 3 2 l Chromate Cr. O 4 l Dichromate Cr 2 O 72 l Silicate Si. O 32 -

3 - ions l Phosphate 3 - PO 4 3 l Phosphite PO 3 1+ ion l Ammonium NH 41+

Adding Hydrogen to Polyatomics l Hydrogen ions are 1+ l Attach to other polyatomic ionschanges charge by one l Sulfate SO 42 l Hydrogen sulfate HSO 41 l Phosphate PO 43 l Hydrogen phosphate HPO 42 l Dihydrogen phosphate H 2 PO 41 -

Ions in Ionic Compounds

Naming Binary Ionic Compounds Binary Compounds - 2 elements. l Ionic - a cation and an anion. l The name is just the names of the ions. l Cation first anion second l Easy with Group A elements. l Na. Cl = Na+ Cl- = sodium chloride l Mg. Br 2 = Mg 2+ Br- = magnesium bromide l Na 2 S l

Naming Binary Ionic Compounds The problem comes with the transition metals. l Cation name includes the charge. l The compound must be neutral. l same number of + and – charges. l Use the negative charge to find the charge on the positive ion. l

Naming Binary Ionic Compounds Write the name of Cu. O l Need the charge of Cu l O is 2 l copper must be 2+ l Copper(II) oxide l Name Co. Cl 3 l Cl is 1 - and there are three of them = 3 l Co must be 3+ l Cobalt(III) chloride l

Naming Binary Ionic Compounds Write the name of Cu 2 S. l Since S is 2 -, the Cu 2 must be 2+, so each one is 1+. l copper(I) sulfide l Fe 2 O 3 l Each O is 23 x 2 - = 6 l 2 Fe must = 6+, so each is 3+. l iron(III) oxide l

Naming Binary Ionic Compounds Write the names of the following l KCl l Na 3 N l Cr. N l Sc 3 P 2 l Pb. O 2 l Na 2 Se l

Ternary Ionic Compounds Will have polyatomic ions l At least three elements (3 capital letters) l Still just name the ions l l Na. NO 3 l Ca. SO 4 l Cu. SO 3

Ternary Ionic Compounds l (NH 4)2 O l Fe(OH)3 l Li. CN l (NH 4)2 CO 3 l Ni. PO 4

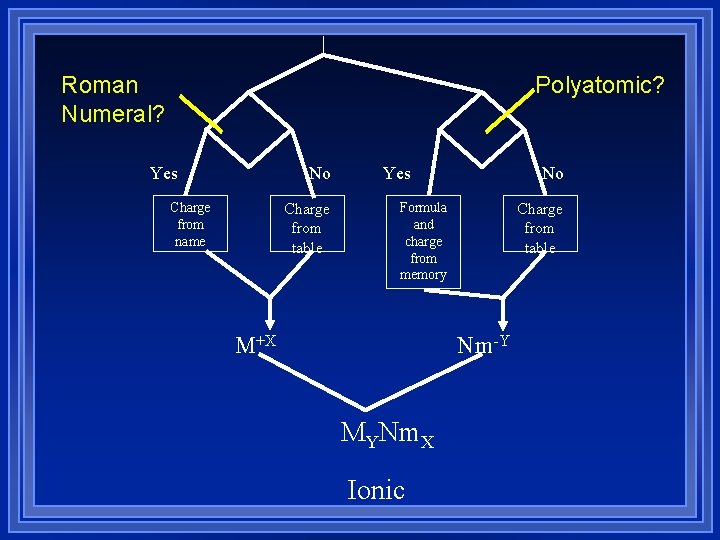
Writing Formulas The charges have to add up to zero. l Get charges on pieces. l Cations from name or periodic table. l Anions from periodic table or polyatomic. l Balance the charges by adding subscripts. l Put polyatomics in parenthesis if there is more than one of them l

Writing Formulas Write the formula for calcium chloride. l Calcium is Ca 2+ l Chloride is Cl 1 l Ca 2+ Cl 1 - would have a 1+ charge. l Need another Cl 1 l Ca 2+ Cl 21 l

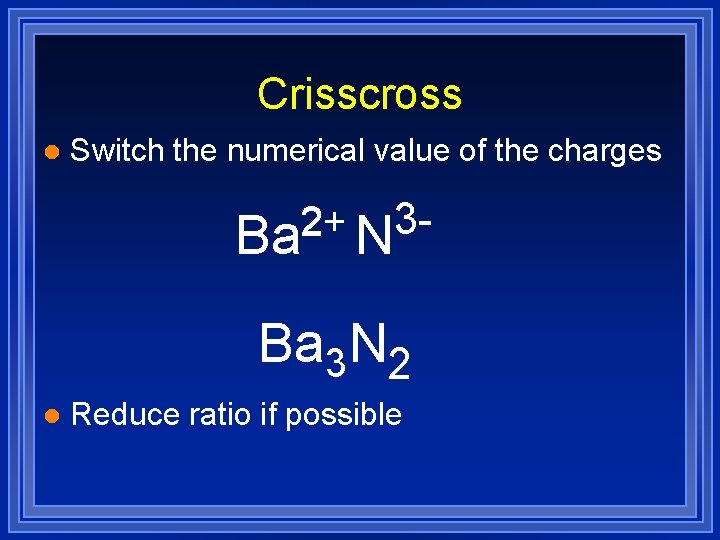
Crisscross l Switch the numerical value of the charges 3 32 2+ Ba N Ba 3 N 2 l Reduce ratio if possible

Write the formulas for these Lithium sulfide l tin (II) oxide l tin (IV) oxide l Copper (II) sulfate l Iron (III) phosphide l gallium nitrate l Iron (III) sulfide l ammonium sulfide l

Write the formulas for these Ammonium chloride l barium nitrate l

Polyatomic? Roman Numeral? Yes No Charge from name Charge from table Yes No Formula and charge from memory M+X Charge from table Nm-Y MYNm. X Ionic

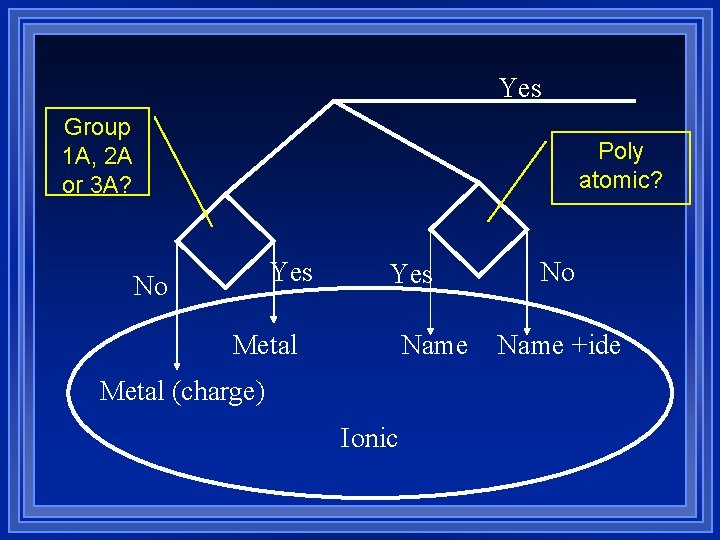
Yes Group 1 A, 2 A or 3 A? Poly atomic? Yes No Yes Metal Name Metal (charge) Ionic No Name +ide

Things to look for If cations have (), the number is their charge. Not how many. l If anions end in -ide they are probably off the periodic table (Monoatomic) l If anion ends in -ate or -ite it is polyatomic l The positive piece always gets written first l Hydrogen- it depends on where it’s at – If it is second, it’s a nonmetal -hydride l

Molecular Compounds Writing names and Formulas

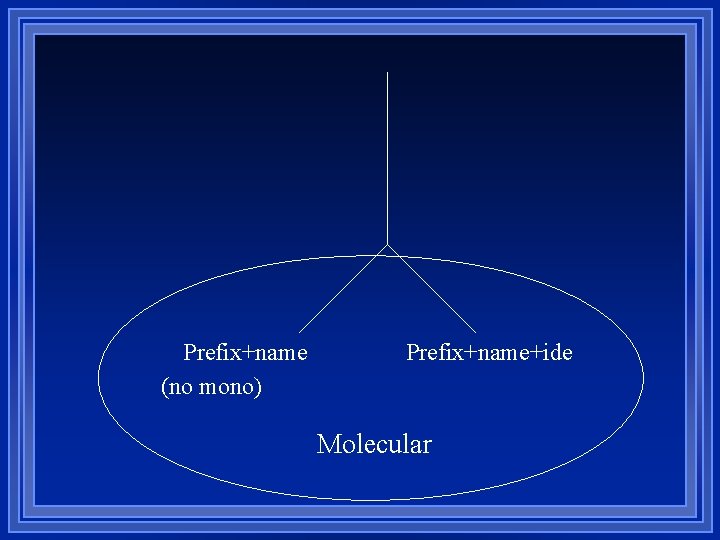
Molecular compounds made of just nonmetals l smallest piece is a molecule l can’t be held together because of opposite charges. l can’t use charges to figure out how many of each atom l

Easier l Ionic compounds use charges to determine how many of each. – Have to figure out charges. – Have to figure out numbers. l Molecular compounds name tells you the number of atoms. l Uses prefixes to tell you the number

Prefixes 1 monol 2 dil 3 tril 4 tetral 5 pental 6 hexal 7 heptal 8 octal 9 nonal 10 decal

Naming l To write the name write two words Prefix name -ide Exception - we don’t write mono- if there is only one of the first element. l No ao oo double vowels when writing name, io, oi, and ai are okay. l

Name These N 2 O l NO 2 l Cl 2 O 7 l CBr 4 l CO 2 l Ba. Cl 2 l

Write formulas for these diphosphorus pentoxide l tetraiodine nonoxide l sulfur hexaflouride l nitrogen trioxide l Carbon tetrahydride l phosphorus trifluoride l aluminum chloride l diagram l

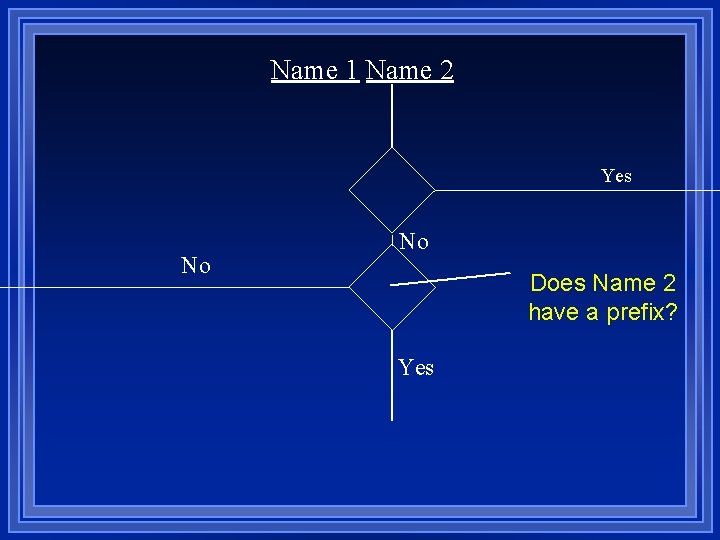
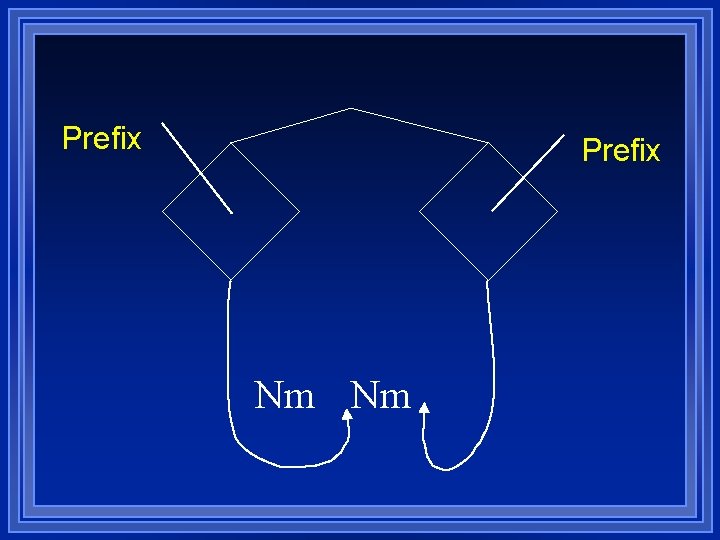
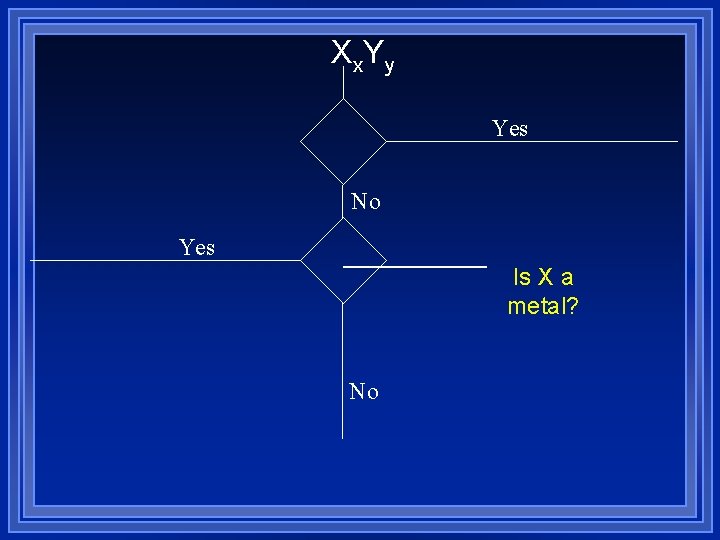
Name 1 Name 2 Yes No No Does Name 2 have a prefix? Yes

Prefix Nm Nm

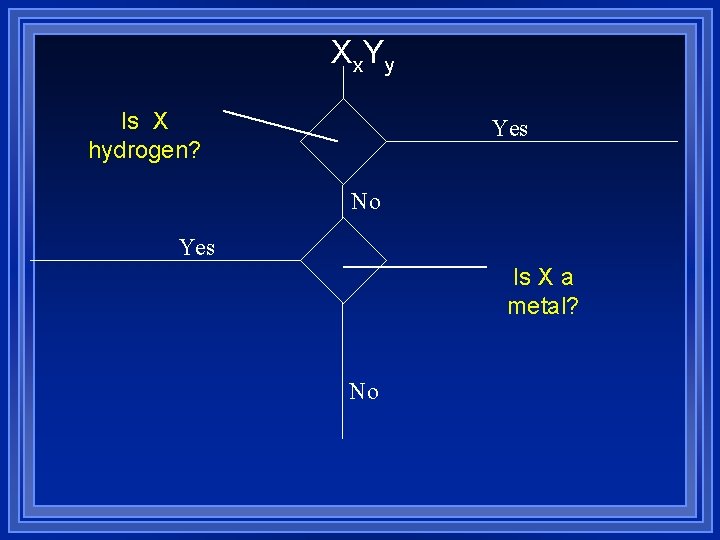
Xx. Yy Yes No Yes Is X a metal? No

Prefix+name (no mono) Prefix+name+ide Molecular

Acids Writing names and Formulas

Acids Compounds that give off hydrogen ions when dissolved in water. l Must have H in them. l will always be some H next to an anion. l The anion determines the name. l

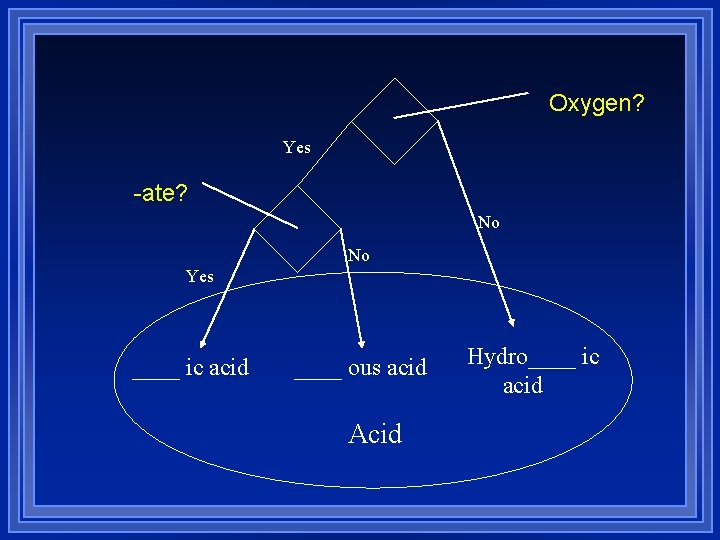
Naming acids If the anion attached to hydrogen is ends in -ide, put the prefix hydro- and change -ide to -ic acid l HCl - hydrogen ion and chloride ion l hydrochloric acid l H 2 S hydrogen ion and sulfide ion l hydrosulfuric acid l

Naming Acids If the anion has oxygen in it l it ends in -ate or -ite l change the suffix -ate to -ic acid l HNO 3 Hydrogen and nitrate ions l Nitric acid l change the suffix -ite to -ous acid l HNO 2 Hydrogen and nitrite ions l Nitrous acid l

Name these HF l H 3 P l H 2 SO 4 l H 2 SO 3 l HCN l H 2 Cr. O 4 l

Writing Formulas Hydrogen will always be first l name will tell you the anion l make the charges cancel out. l Starts with hydro- no oxygen, -ide l no hydro, -ate comes from -ic, -ite comes from -ous l

Write formulas for these hydroiodic acid l acetic acid l carbonic acid l phosphorous acid l hydrobromic acid l diagram l

Name 1 Name 2 Is Name 2 acid? No Yes No Does Name 2 have a prefix? Yes

No Hydro- ? No Yes Charge from table -ic acid? Yes -ate Nm-Y HYNm No -ite

Xx. Yy Is X hydrogen? Yes No Yes Is X a metal? No

Oxygen? Yes -ate? No No Yes ____ ic acid ____ ous acid Acid Hydro____ ic acid

38. Name these acids a) H 2 C 2 O 4 b) HF c) HCl. O 2 d) H 2 CO 3 39. Write formulas for these compounds a) nitrous acid b) hydroselenic acid c) phosphoric acid d) acetic acid 40. 43. Name these compounds a) Al. F 3 b) Sn. O 2 c) Fe(C 2 H 3 O 2)3 d) KHSO 4 e) Ca. H 2 f) HCl. O 3 g) Hg 2 Br 2 h) H 2 Cr. O 4 41. 44. Write formulas for these a) Phosphorus pentabromide b) Carbon chloride c) potassium permanganate

43. Name these compounds a) Al. F 3 b) Sn. O 2 c) Fe(C 2 H 3 O 2)3 d) KHSO 4 e) Ca. H 2 f) HCl. O 3 g) Hg 2 Br 2 h) H 2 Cr. O 4 44. Write formulas for these a) Phosphorus pentabromide b) Carbon chloride c) potassium permanganate d) Calcium hydrogen carbonate e) dichlorine heptoxide f) trisilicon tetrahydride g) sodium dihydrogen phosphate

Summary Periodic table – Grouped by properties l Metals- make cations – 2 types those with () and those without l Nonmetals make anions – Three types • Without O -ide • With O -ite and -ate l Only electrons can move to make ions l

Summary l l l l l Make all the decisions. First determine type of compound Then figure out name or formula Acid = H to start Metal = Ionic No H, No metal = molecular Only molecular get prefixes Roman numeral is NOT how many Hydro means no O