Naming and Writing Ionic Formulas Naming Ionic Compounds




- Slides: 22

Naming and Writing Ionic Formulas

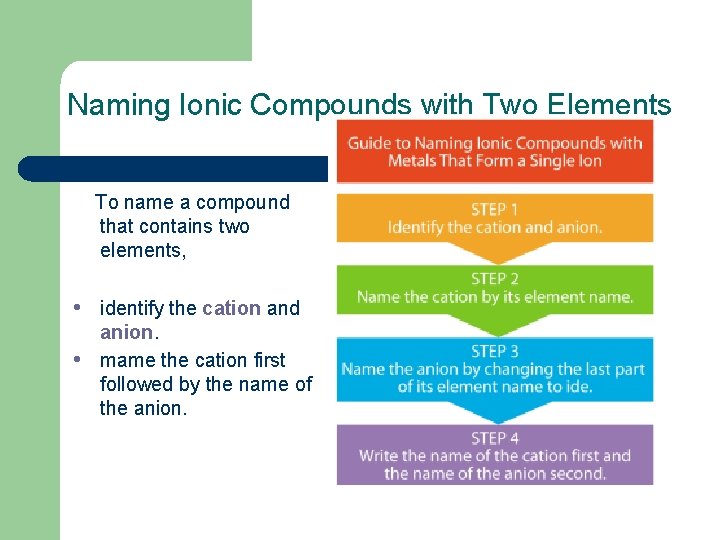
Naming Ionic Compounds with Two Elements To name a compound that contains two elements, • identify the cation and • anion. mame the cation first followed by the name of the anion.

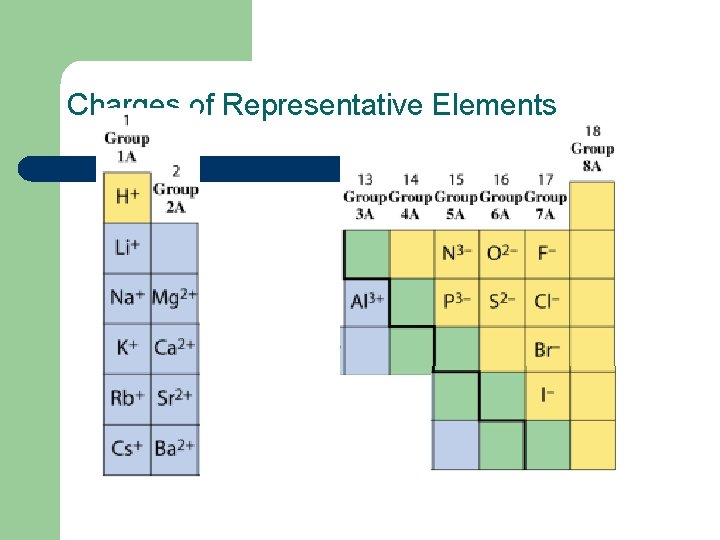
Charges of Representative Elements

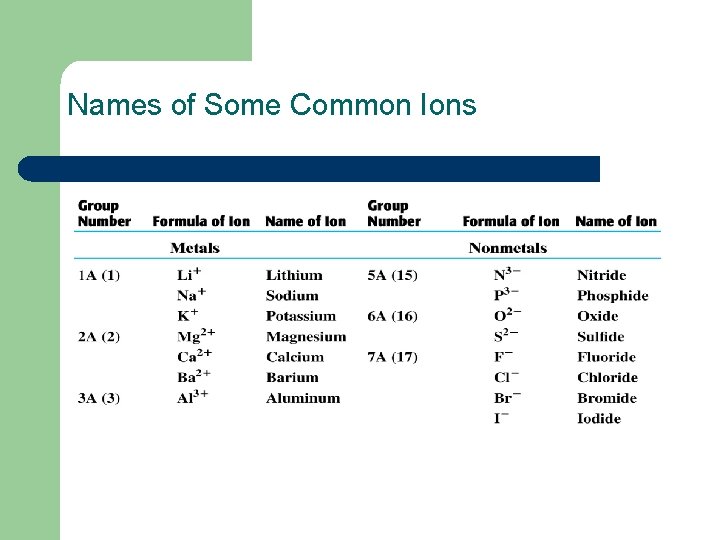
Names of Some Common Ions

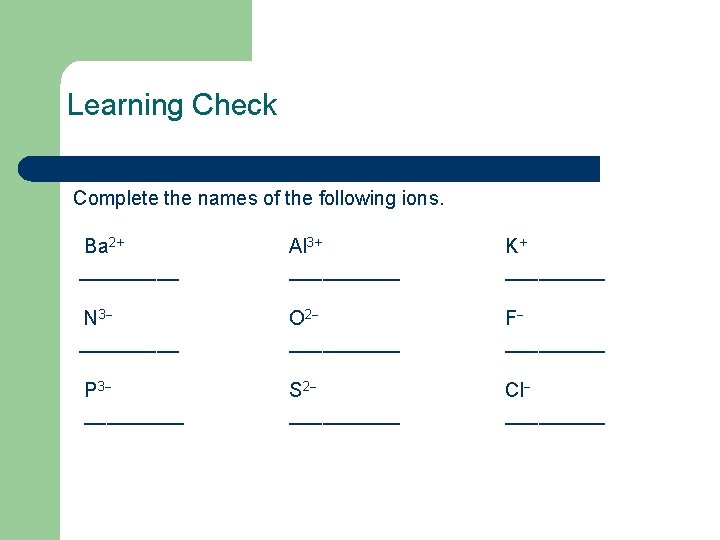
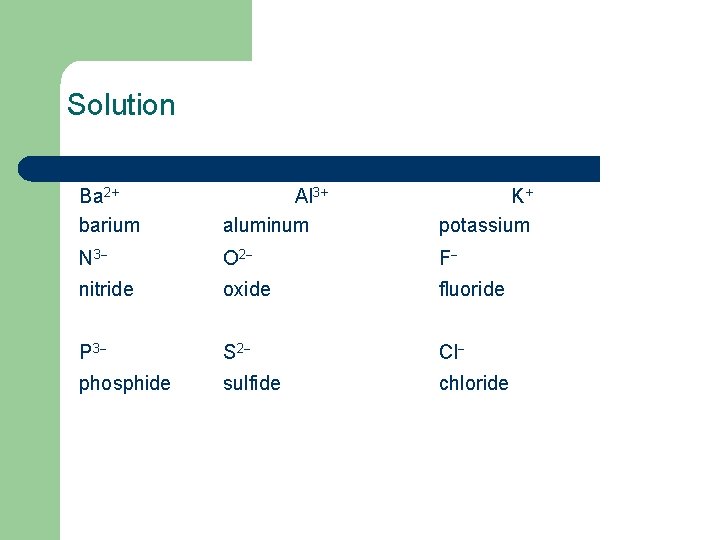
Learning Check Complete the names of the following ions. Ba 2+ _____ Al 3+ _____ K+ _____ N 3 _____ O 2 _____ F _____ P 3 _____ S 2 _____ Cl _____

Solution Ba 2+ barium Al 3+ aluminum K+ potassium N 3 O 2 F nitride oxide fluoride P 3 S 2 Cl phosphide sulfide chloride

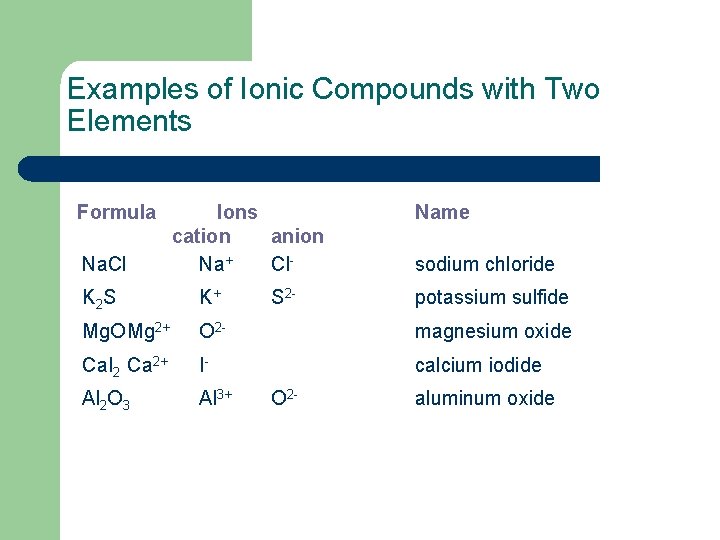
Examples of Ionic Compounds with Two Elements Formula Na. Cl Ions cation anion Na+ Cl. S 2 - Name sodium chloride K 2 S K+ Mg. OMg 2+ O 2 - magnesium oxide Ca. I 2 Ca 2+ I- calcium iodide Al 2 O 3 Al 3+ O 2 - potassium sulfide aluminum oxide

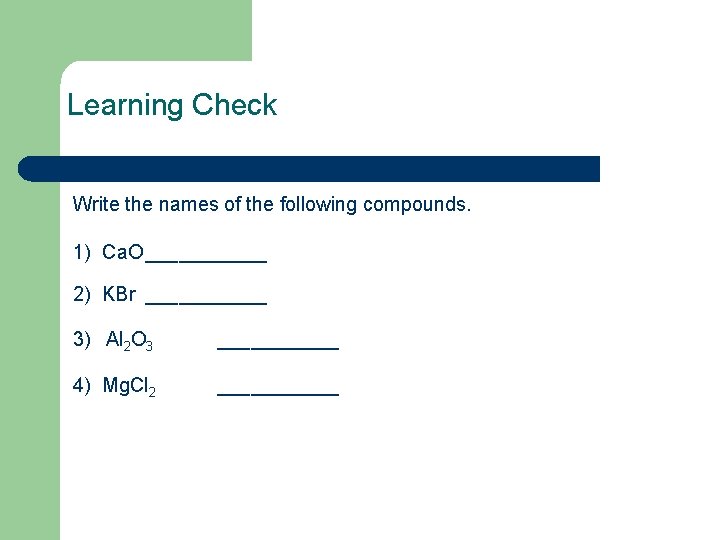
Learning Check Write the names of the following compounds. 1) Ca. O ______ 2) KBr ______ 3) Al 2 O 3 ______ 4) Mg. Cl 2 ______

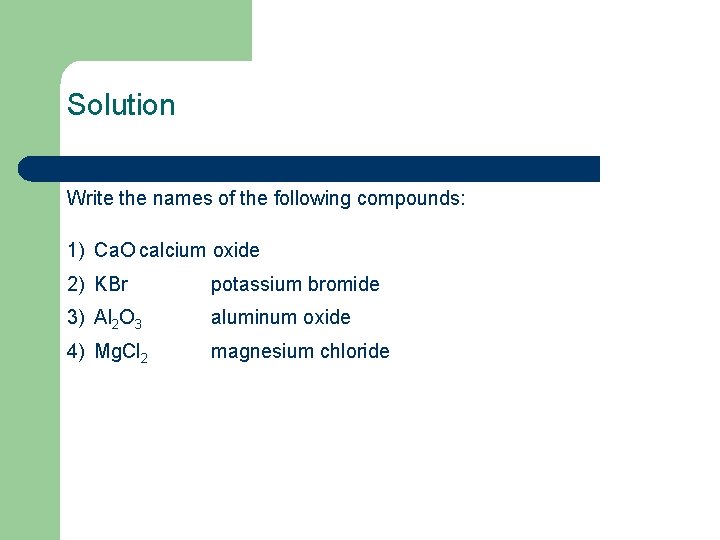
Solution Write the names of the following compounds: 1) Ca. O calcium oxide 2) KBr potassium bromide 3) Al 2 O 3 aluminum oxide 4) Mg. Cl 2 magnesium chloride

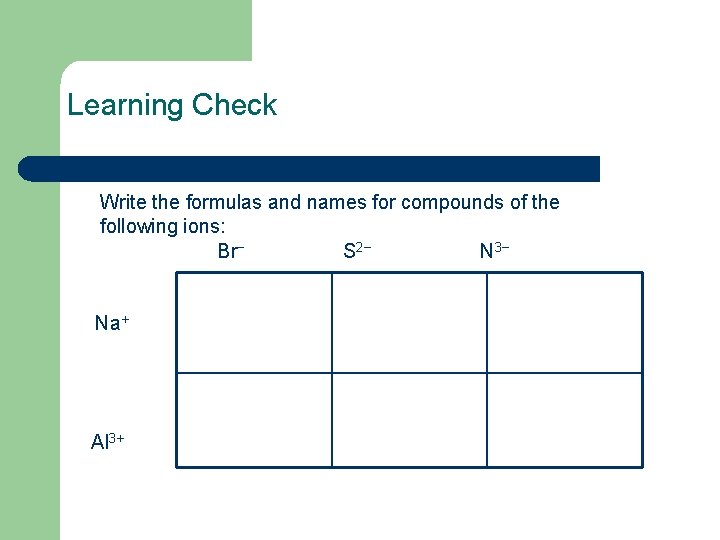
Learning Check Write the formulas and names for compounds of the following ions: Br− S 2− N 3− Na+ Al 3+

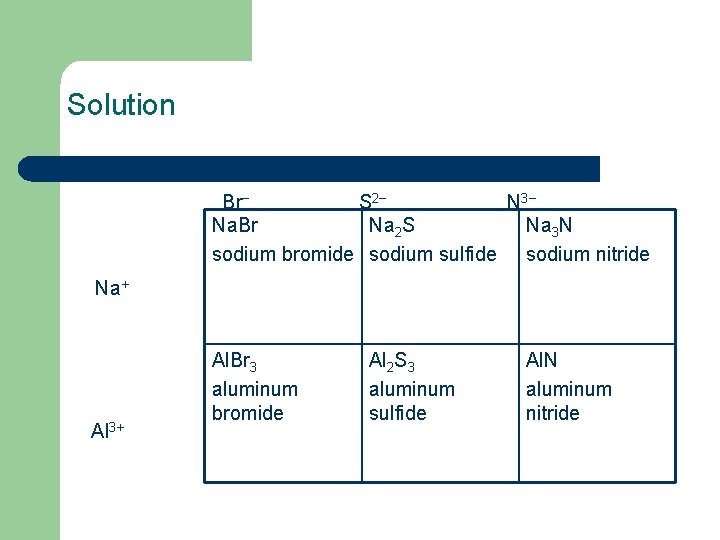
Solution Br− S 2− N 3− Na. Br Na 2 S Na 3 N sodium bromide sodium sulfide sodium nitride Na+ Al 3+ Al. Br 3 aluminum bromide Al 2 S 3 aluminum sulfide Al. N aluminum nitride

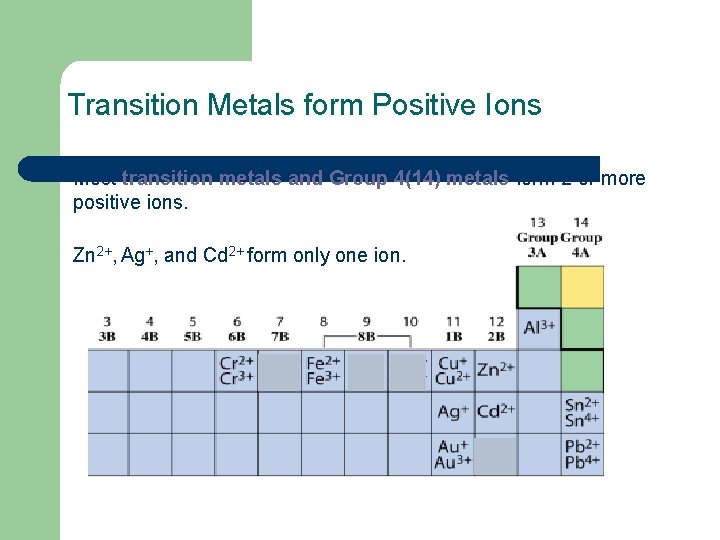
Transition Metals form Positive Ions Most transition metals and Group 4(14) metals form 2 or more positive ions. Zn 2+, Ag+, and Cd 2+ form only one ion.

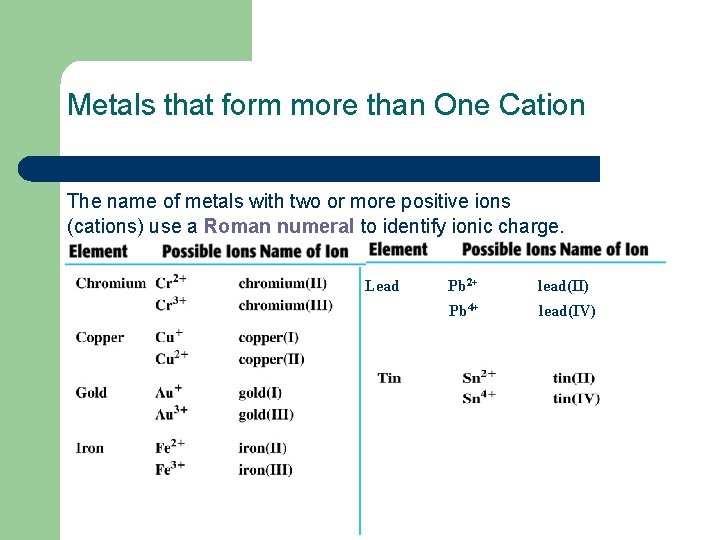
Metals that form more than One Cation The name of metals with two or more positive ions (cations) use a Roman numeral to identify ionic charge. Lead Pb 2+ lead(II) Pb 4+ lead(IV)

Naming Ionic Compounds with Variable Charge Metals

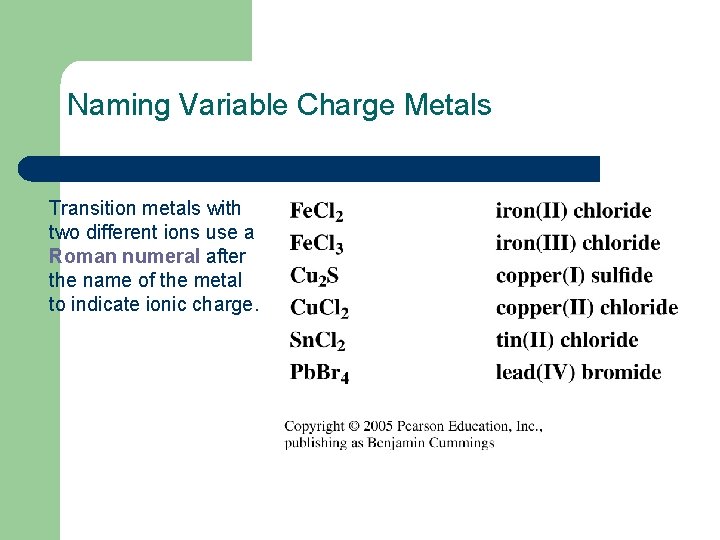
Naming Variable Charge Metals Transition metals with two different ions use a Roman numeral after the name of the metal to indicate ionic charge.

Naming Fe. Cl 2 To name Fe. Cl 2 1. Determine the charge of the cation using the charge of the anion (Cl-). Fe ion + 2 Cl- = Fe ion + 2 - = 0 Fe ion = 2+ 2. Name the cation by the element name and add a Roman numeral in parenthesis to show its charge. Fe 2+ = iron(II) 3. Write the anion with an ide ending. Fe. Cl 2 = iron(II) chloride

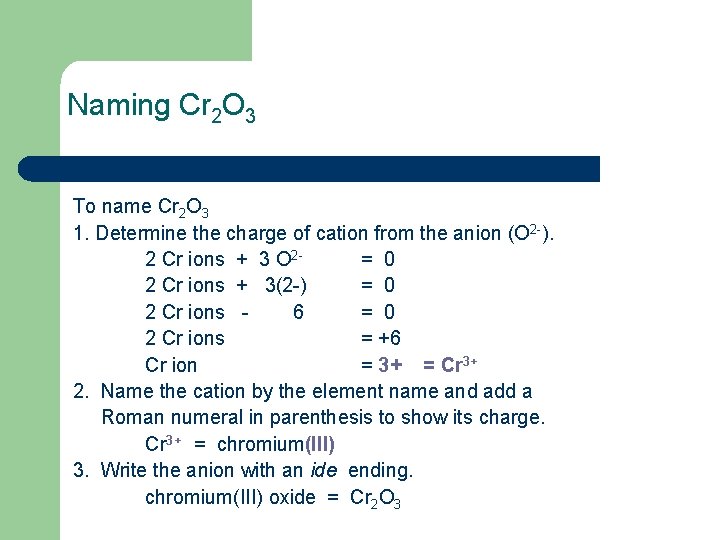
Naming Cr 2 O 3 To name Cr 2 O 3 1. Determine the charge of cation from the anion (O 2 -). 2 Cr ions + 3 O 2= 0 2 Cr ions + 3(2 -) = 0 2 Cr ions 6 = 0 2 Cr ions = +6 Cr ion = 3+ = Cr 3+ 2. Name the cation by the element name and add a Roman numeral in parenthesis to show its charge. Cr 3+ = chromium(III) 3. Write the anion with an ide ending. chromium(III) oxide = Cr 2 O 3

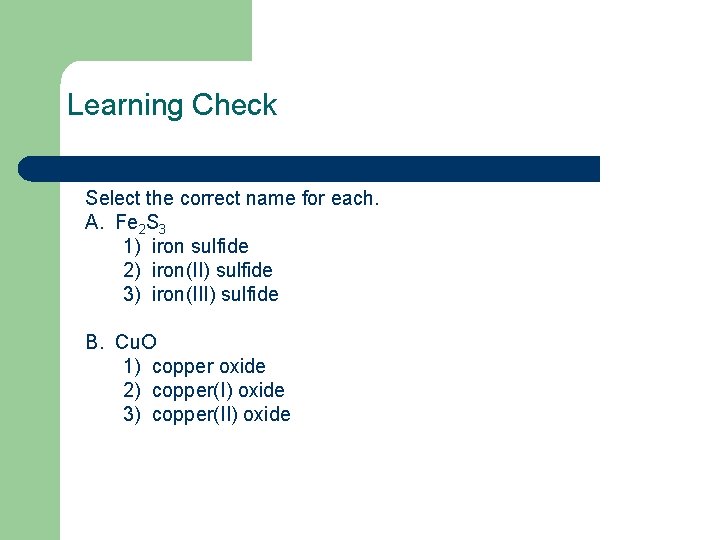
Learning Check Select the correct name for each. A. Fe 2 S 3 1) iron sulfide 2) iron(II) sulfide 3) iron(III) sulfide B. Cu. O 1) copper oxide 2) copper(I) oxide 3) copper(II) oxide

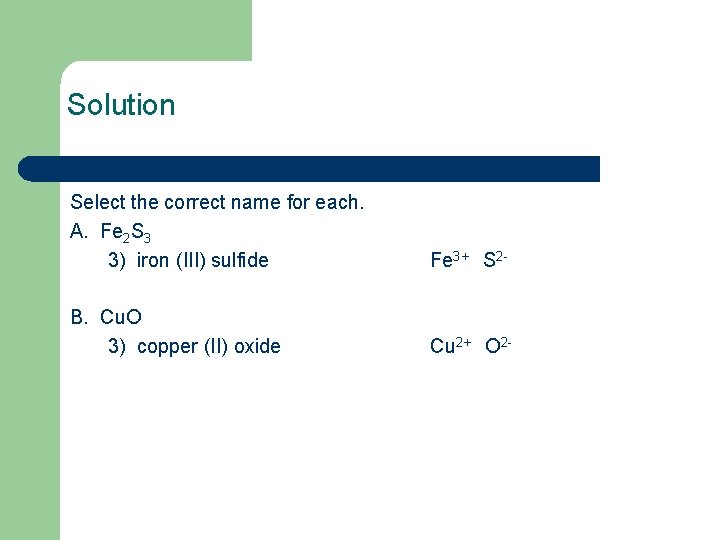
Solution Select the correct name for each. A. Fe 2 S 3 3) iron (III) sulfide Fe 3+ S 2 - B. Cu. O 3) copper (II) oxide Cu 2+ O 2 -

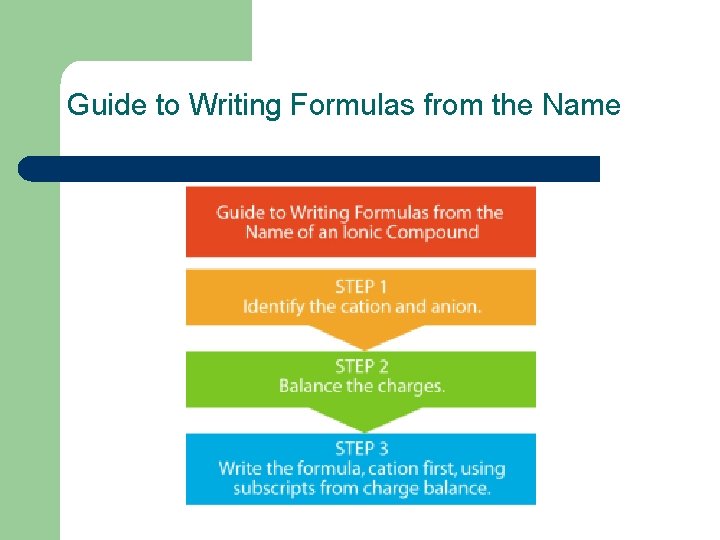
Guide to Writing Formulas from the Name

Writing Formulas Write a formula for potassium sulfide. 1. Identify the cation and anion. potassium = K+ sulfide = S 2− 2. Balance the charges. K+ S 2− K+ 2(1+) + 2(1 -) = 0 3. 2 K+ and 1 S 2− = K 2 S

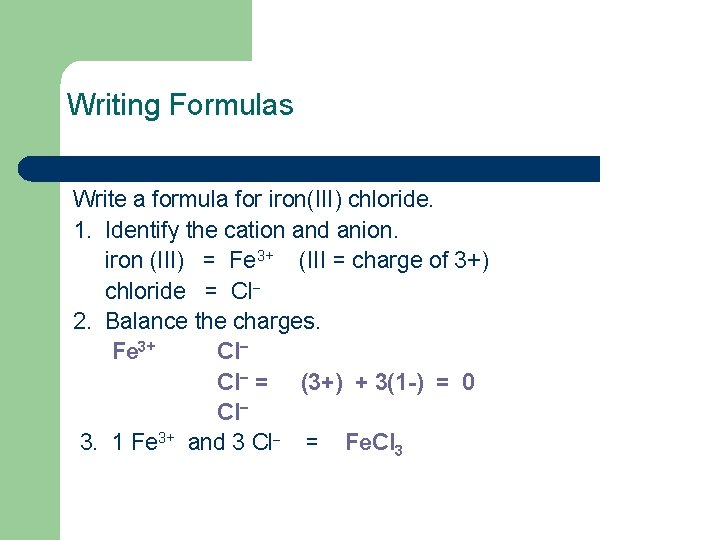
Writing Formulas Write a formula for iron(III) chloride. 1. Identify the cation and anion. iron (III) = Fe 3+ (III = charge of 3+) chloride = Cl− 2. Balance the charges. Fe 3+ Cl− = (3+) + 3(1 -) = 0 Cl− 3. 1 Fe 3+ and 3 Cl− = Fe. Cl 3