Writing and Naming Chemical Formulas for Ionic Compounds











- Slides: 17

Writing and Naming Chemical Formulas for Ionic Compounds: Part 1 Science 10

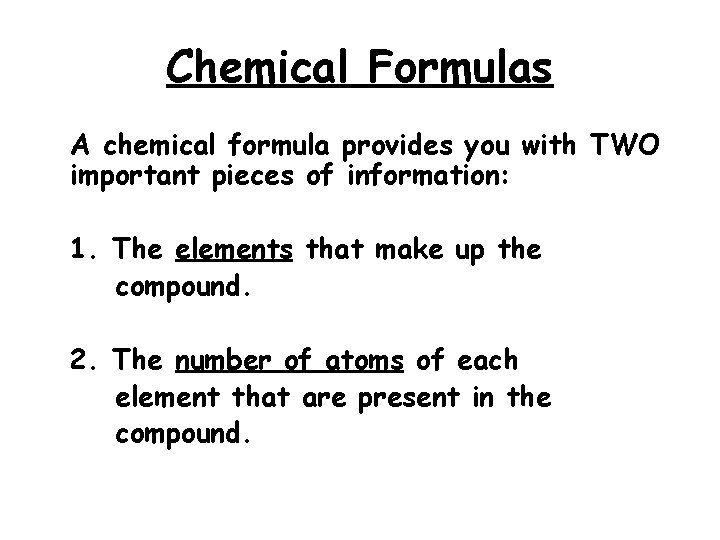
Chemical Formulas A chemical formula provides you with TWO important pieces of information: 1. The elements that make up the compound. 2. The number of atoms of each element that are present in the compound.

Number of Atoms • In an ionic compound, the formula represents the ratio of each atom present in a compound. – Example: In the compound Li 2 O, there are 2 lithium atoms for every 1 oxygen atom.

How many atoms are shown? • Example 1: Li. NO 3 – 5 atoms • Example 2: Mg(OH)2 - 5 atoms • Example 3: (NH 4)3 PO 4 • - 20 atoms

Valence Numbers • We have already used Lewis structures to determine the chemical formulas of compounds, but this can get to be quite tedious (especially with large compounds). • We will now use the shorthand method of determining chemical formulas ~ using combining capacities (AKA: valence numbers).

Valence Numbers (Continued) • A positive valence number indicates the loss of electrons. • A negative valence number indicates the gaining of electrons. • The number next to the + or – indicates HOW MANY electrons are lost or gained to achieve a stable octet, and therefore form a compound.

Writing Chemical Formulas for Simple Ionic Compounds 1. Write the atom’s symbols with their combining capacities (as superscripts). 2. Crisscross the combining capacities, and write them (WITHOUT the + or -) as subscripts. 3. Write the final chemical formula. - If there any 1’s, remove them. - If the subscripts are multiples of each other, reduce them.

Examples: 1. calcium fluoride Ca. F 2 2. zinc oxide Zn. O 3. Potassium sulphide K 2 S 4. barium phosphide Ba 3 P

Naming Simple Ionic Compounds 1. Write the name of the metal first. 2. Followed by the name of the nonmetal). 3. Remove the ending of the non-metal and add “ide”.

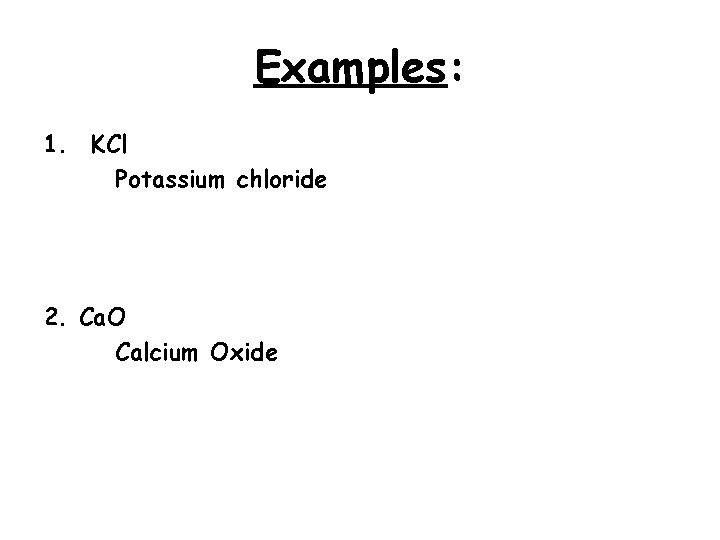
Examples: 1. KCl Potassium chloride 2. Ca. O Calcium Oxide

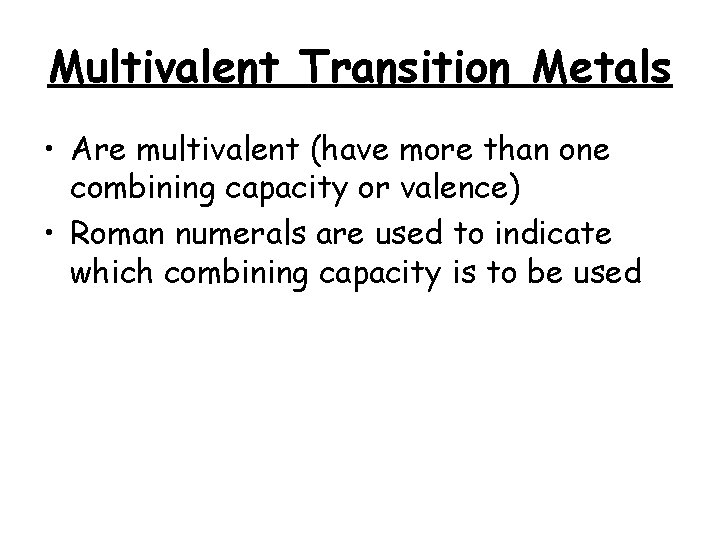
Multivalent Transition Metals • Are multivalent (have more than one combining capacity or valence) • Roman numerals are used to indicate which combining capacity is to be used

Writing Chemical Formulas for Compounds Containing Transition Metals 1. Write the atom’s symbols with their combining capacities (as superscripts). - Please note that the combining capacity that you use for the transition metal is indicated by the Roman numeral in the name. 2. Crisscross the combining capacities, and write them (WITHOUT the + or -) as subscripts. 3. Write the final chemical formula. - If there any 1’s, remove them. - If the subscripts are multiples of each other, reduce them.

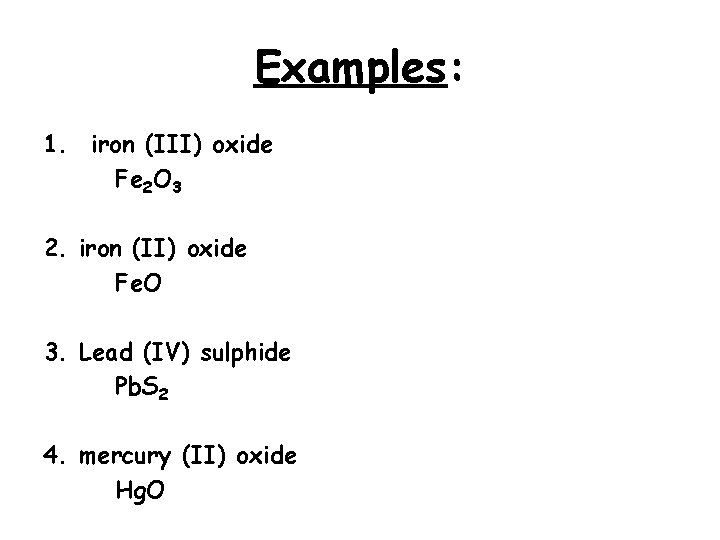
Examples: 1. iron (III) oxide Fe 2 O 3 2. iron (II) oxide Fe. O 3. Lead (IV) sulphide Pb. S 2 4. mercury (II) oxide Hg. O

Naming Multivalent Compounds Step 1: Write the names of the metal and non-metal, leaving a space after the metal for a roman numeral Step 2: Reverse ‘criss cross’, this will give you the ion charge of the metal. The nonmetal charge is always constant (look at periodic table) Step 3: Write formula including roman numeral for metal. **NOTE watch for reducing**

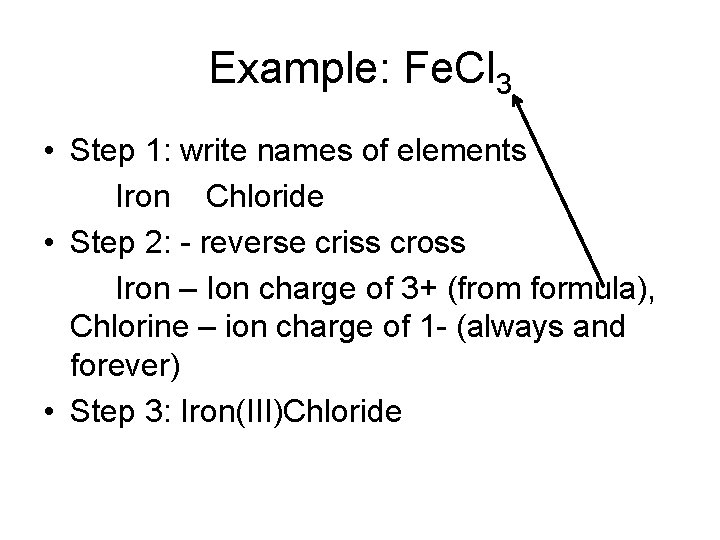
Example: Fe. Cl 3 • Step 1: write names of elements Iron Chloride • Step 2: - reverse criss cross Iron – Ion charge of 3+ (from formula), Chlorine – ion charge of 1 - (always and forever) • Step 3: Iron(III)Chloride

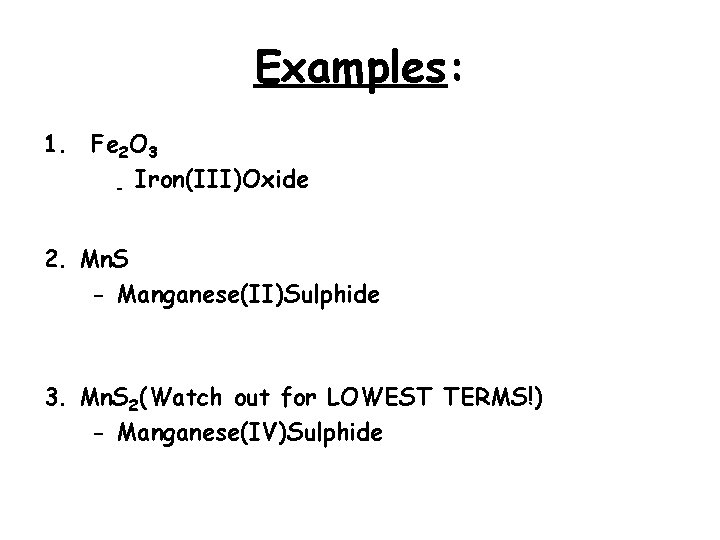
Examples: 1. Fe 2 O 3 - Iron(III)Oxide 2. Mn. S - Manganese(II)Sulphide 3. Mn. S 2(Watch out for LOWEST TERMS!) - Manganese(IV)Sulphide

Try the Magic Formula !!!! 1. Fe 2 O 3 Answer - Iron (III) Oxide 2. Mn. S – watch for reducing – -hint S is always 2 Answer – Manganese (II) Sulphide 3. Mn. S 2 Answer – Manganese (IV) Sulphide – again check for reducing – Sulphide is 2 -, Manganese was reduced from 4+