Naming Writing Formulas For Molecular Ionic Compounds Naming




















- Slides: 61

Naming & Writing Formulas For Molecular & Ionic Compounds

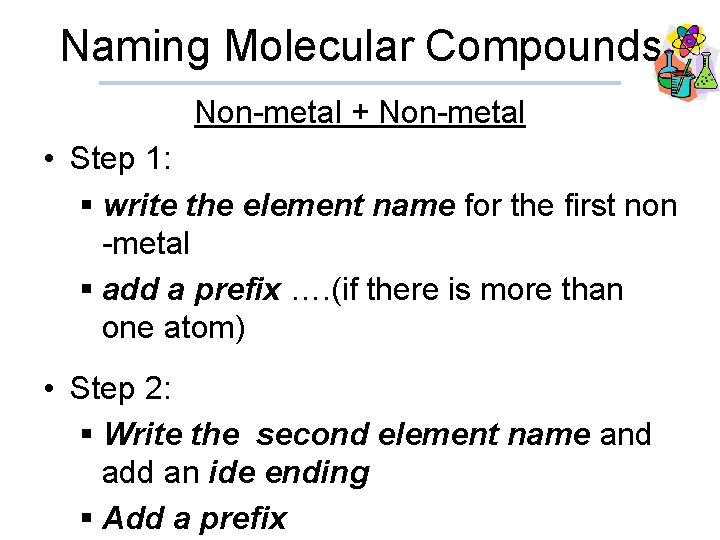
Naming Molecular Compounds Non-metal + Non-metal • Step 1: § write the element name for the first non -metal § add a prefix …. (if there is more than one atom) • Step 2: § Write the second element name and add an ide ending § Add a prefix

Exceptions • DO NOT use any prefixes at all if the first element is hydrogen ……these are acids Prefixes 6= hexa 7= hepta 3= mono di tri 8= octa 4= tetra 9= nona 5= penta 10 = deca 1= 2=

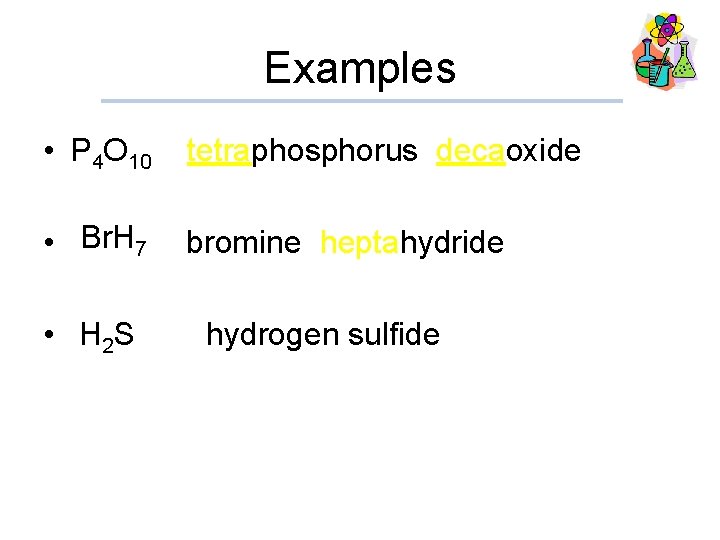
Examples • P 4 O 10 tetraphosphorus decaoxide • Br. H bromine heptahydride 7 • H 2 S hydrogen sulfide

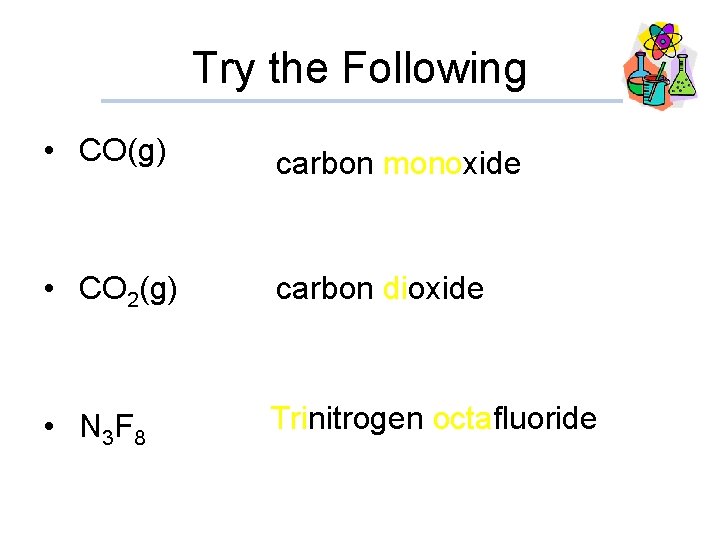
Try the Following • CO(g) carbon monoxide • CO 2(g) carbon dioxide • N 3 F 8 Trinitrogen octafluoride

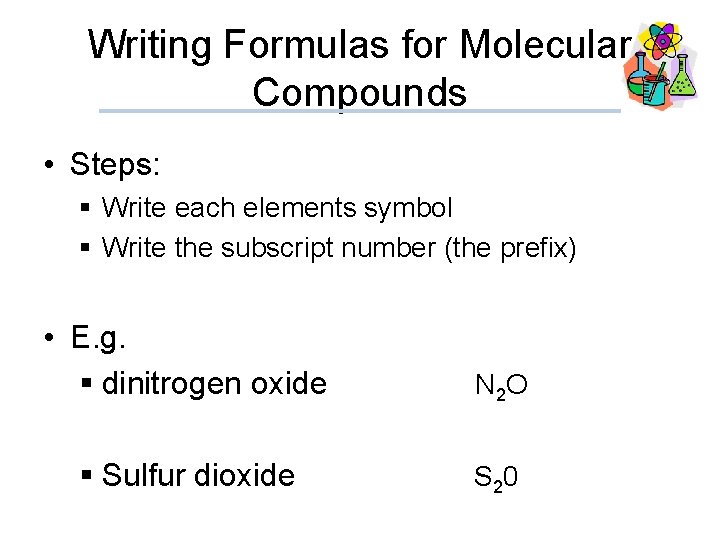
Writing Formulas for Molecular Compounds • Steps: § Write each elements symbol § Write the subscript number (the prefix) • E. g. § dinitrogen oxide N 2 O § Sulfur dioxide S 20

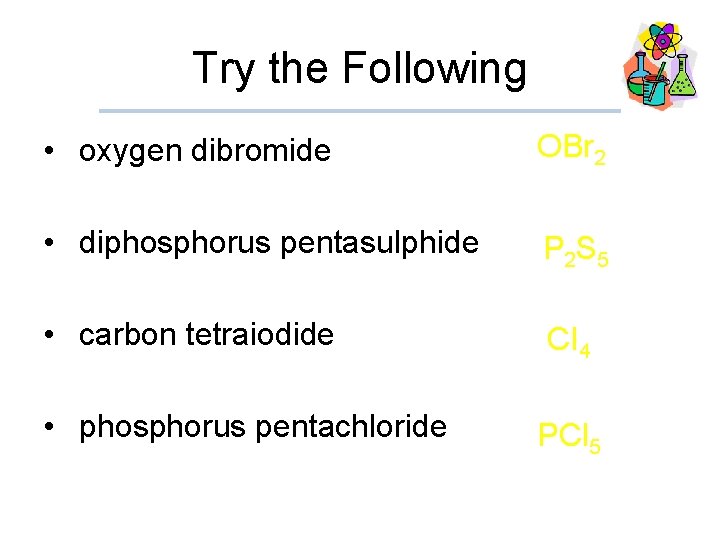
Try the Following • oxygen dibromide OBr 2 • diphosphorus pentasulphide P 2 S 5 • carbon tetraiodide CI 4 • phosphorus pentachloride PCl 5

Molecular Compounds that Must be memorized !!! NH 3 ( g) = ammonia H 2 O ( l) = water H 2 S ( g) = hydrogen sulphide CH 4 ( g) = methane CH 3 OH ( l) = methanol C 2 H 6 ( g) = ethane C 2 H 5 OH ( l) = ethanol C 6 H 12 O 6 ( s) = glucose C 12 H 22 O 11 ( s) = sucrose O 3 ( g) = ozone H 2 O 2 ( l) = hydrogen peroxide

Naming Binary Ionic Compounds Metal + non-metal • DO NOT USE PREFIXES • Steps 1. Write the metal 1 st 2. Write the non-metal 2 nd with an ide ending

E. g. Na. F sodium fluoride Na 2 S sodium sulphide two sodium ions are bonded with one sulphide ion… this doesn’t matter for naming ionic compound

Try the Following • • Li. F KCl Be. S Rb 3 P Mg. F 2 Na 2 O Cs. Br lithium fluoride potassium chloride beryllium sulphide rubidium phosphide magnesium fluoride sodium oxide cesium bromide

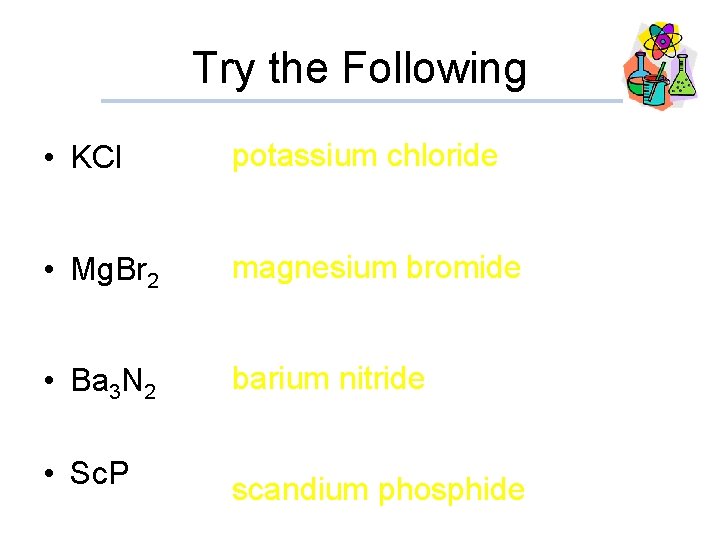
Try the Following • KCl potassium chloride • Mg. Br 2 magnesium bromide • Ba 3 N 2 barium nitride • Sc. P scandium phosphide

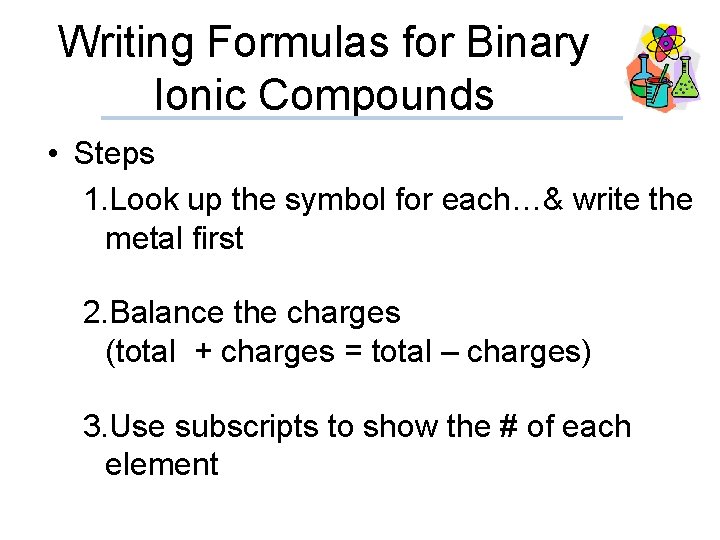
Writing Formulas for Binary Ionic Compounds • Steps 1. Look up the symbol for each…& write the metal first 2. Balance the charges (total + charges = total – charges) 3. Use subscripts to show the # of each element

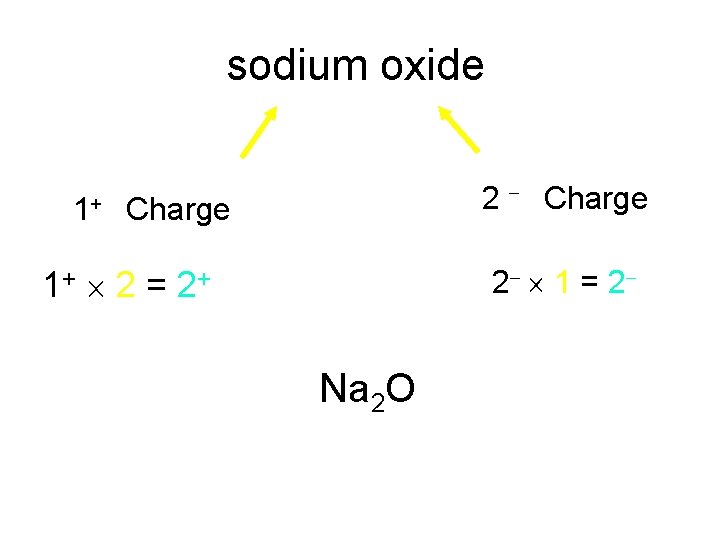
sodium oxide 2 Charge 1+ Charge 2 1 = 2 1+ 2 = 2+ Na 2 O

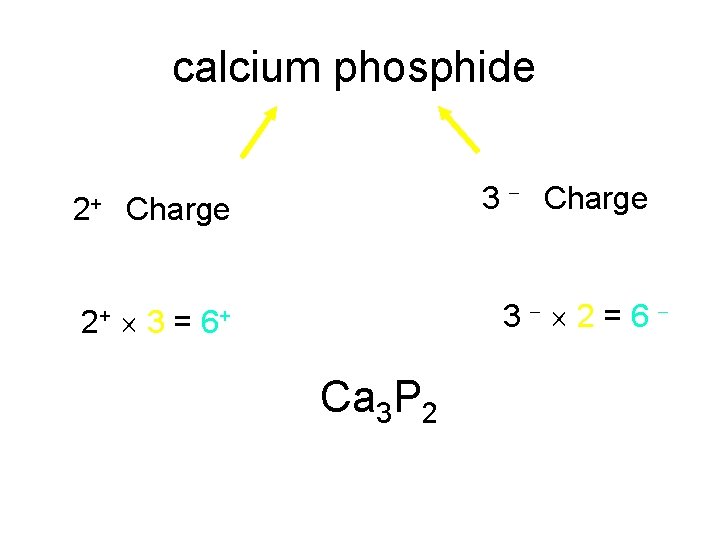
calcium phosphide 3 Charge 2+ Charge 3 2 = 6 2+ 3 = 6+ Ca 3 P 2

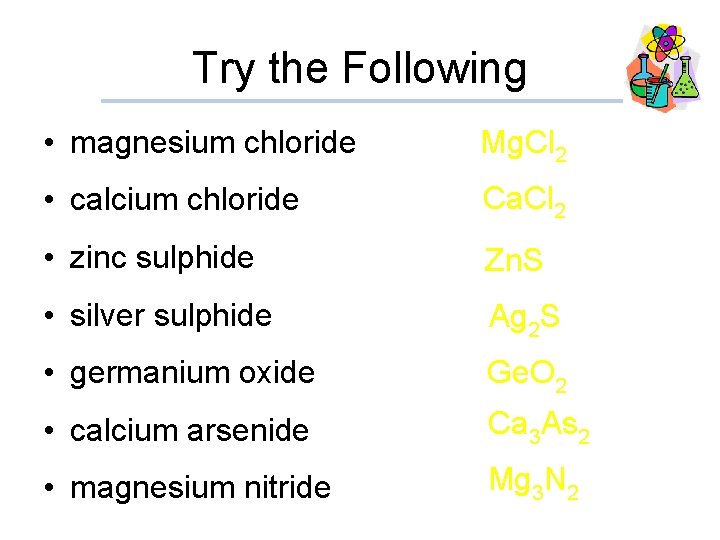
Try the Following • magnesium chloride • calcium chloride Mg. Cl 2 Ca. Cl 2 • zinc sulphide Zn. S • silver sulphide Ag 2 S • germanium oxide Ge. O 2 • calcium arsenide • magnesium nitride Ca 3 As 2 Mg 3 N 2

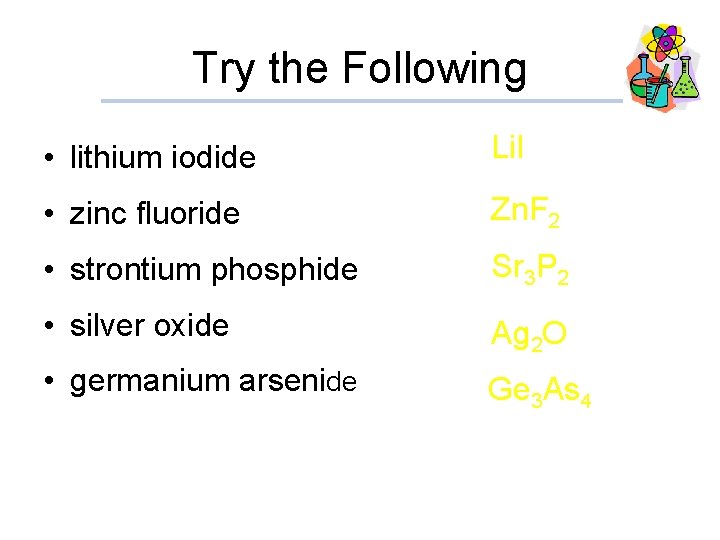
Try the Following • lithium iodide Li. I • zinc fluoride Zn. F 2 • strontium phosphide Sr 3 P 2 • silver oxide Ag 2 O • germanium arsenide Ge 3 As 4

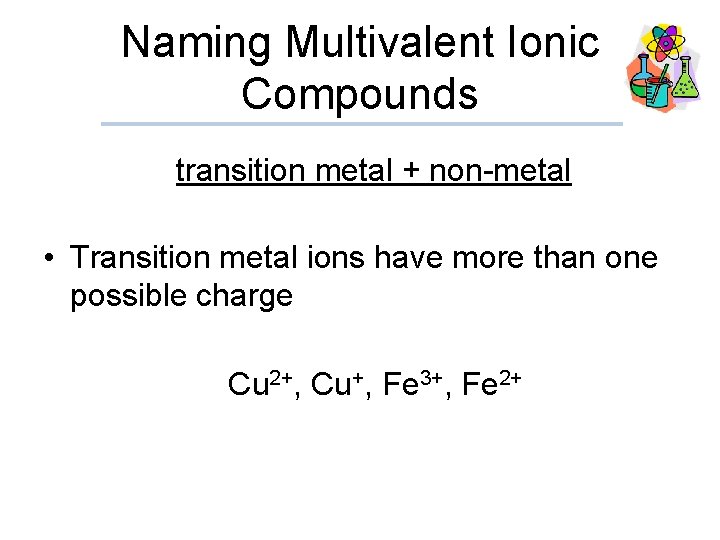
Naming Multivalent Ionic Compounds transition metal + non-metal • Transition metal ions have more than one possible charge Cu 2+, Cu+, Fe 3+, Fe 2+

• Steps 1. Write metal 1 st with the charge in roman numerals Roman Numerals (I, III, IV, V, VII) 2. Write non-metal second remember the charges have to balance

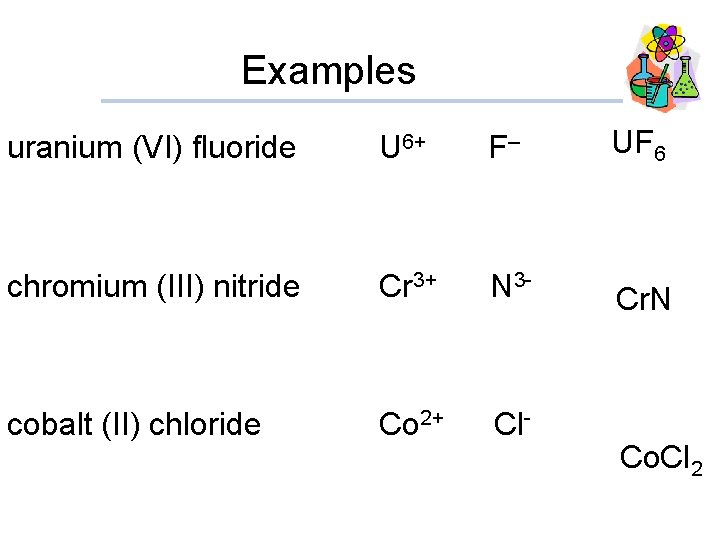
Examples uranium (VI) fluoride U 6+ F– UF 6 chromium (III) nitride Cr 3+ N 3 - Cr. N cobalt (II) chloride Co 2+ Cl- Co. Cl 2

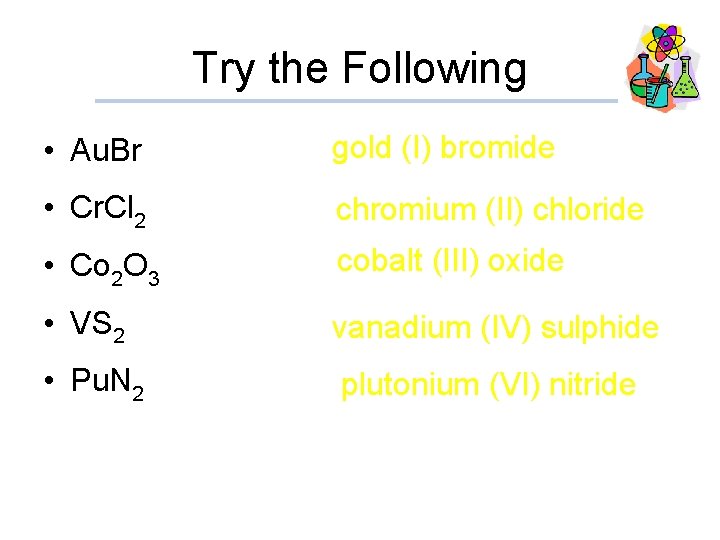
Try the Following • Au. Br gold (I) bromide • Cr. Cl 2 chromium (II) chloride • Co 2 O 3 cobalt (III) oxide • VS 2 vanadium (IV) sulphide • Pu. N 2 plutonium (VI) nitride

Naming Complex Ions Metal + complex ion • Steps: 1. Name the metal ion 2. Name the complex ion E. g. ) PO 43 Note: NH 4+ (ammonium ion) is the only positive complex ion…it will take the place of a metal

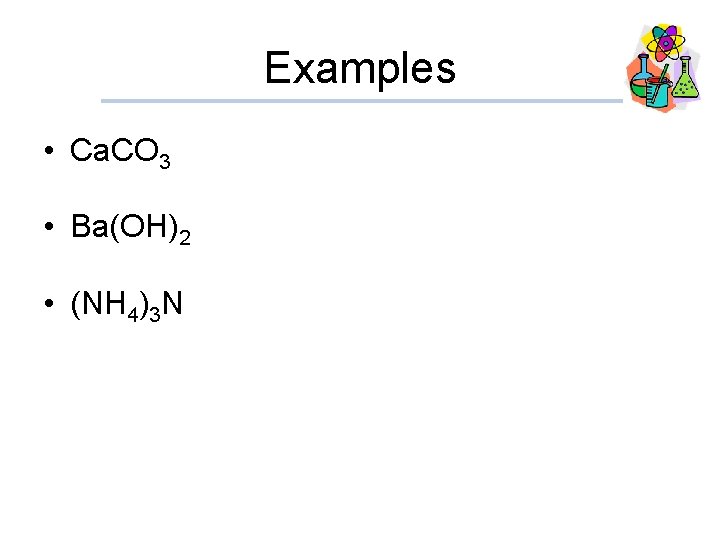
Examples • Ca. CO 3 • Ba(OH)2 • (NH 4)3 N

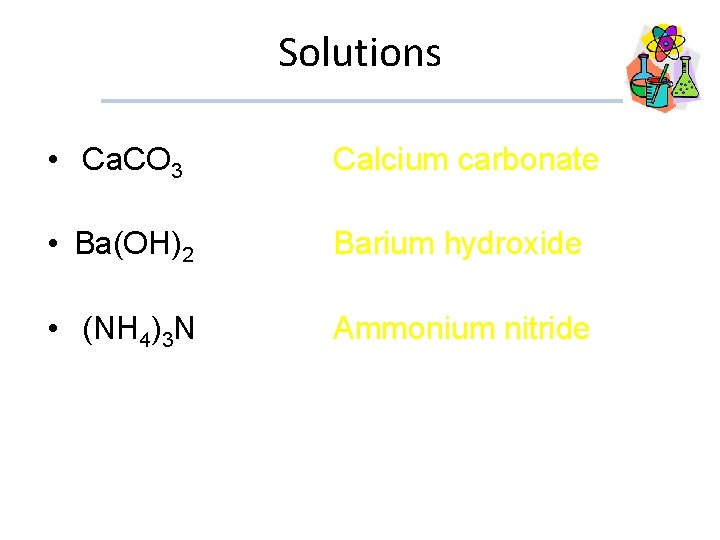
Solutions • Ca. CO 3 Calcium carbonate • Ba(OH)2 • (NH 4)3 N Barium hydroxide Ammonium nitride

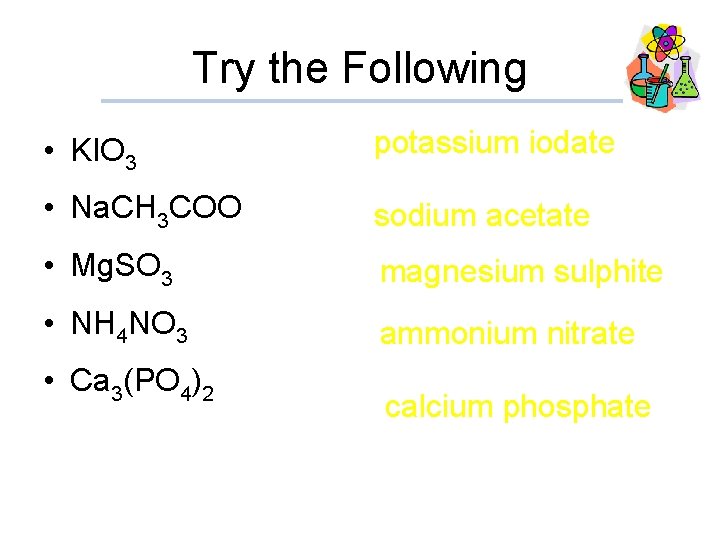
Try the Following • KIO 3 potassium iodate • Na. CH 3 COO sodium acetate • Mg. SO 3 magnesium sulphite • NH 4 NO 3 ammonium nitrate • Ca 3(PO 4)2 calcium phosphate

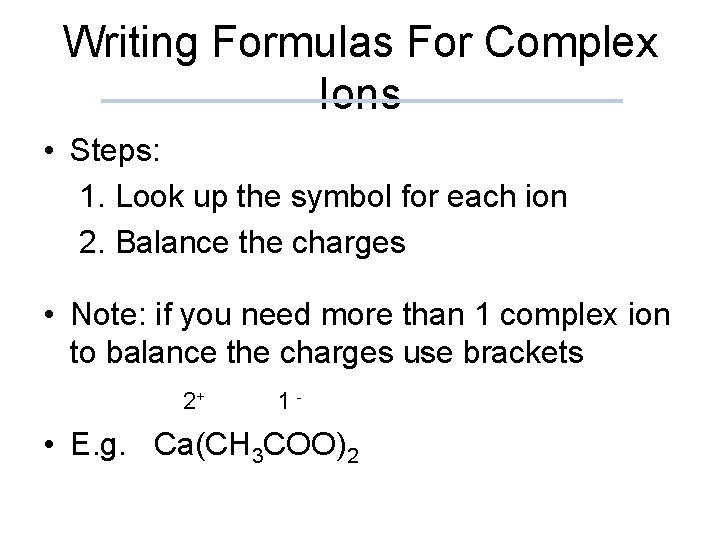
Writing Formulas For Complex Ions • Steps: 1. Look up the symbol for each ion 2. Balance the charges • Note: if you need more than 1 complex ion to balance the charges use brackets 2+ 1 - • E. g. Ca(CH 3 COO)2

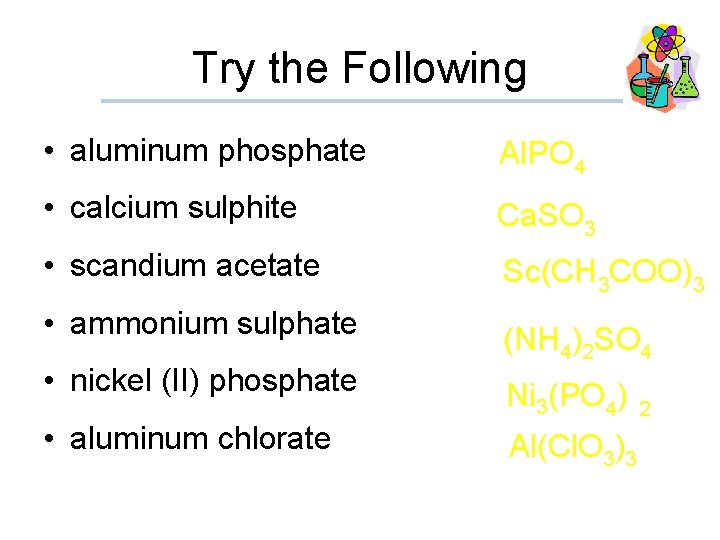
Try the Following • aluminum phosphate Al. PO 4 • calcium sulphite Ca. SO 3 • scandium acetate Sc(CH 3 COO)3 • ammonium sulphate (NH 4)2 SO 4 • nickel (II) phosphate Ni (PO ) 3 • aluminum chlorate 4 2 Al(Cl. O 3)3

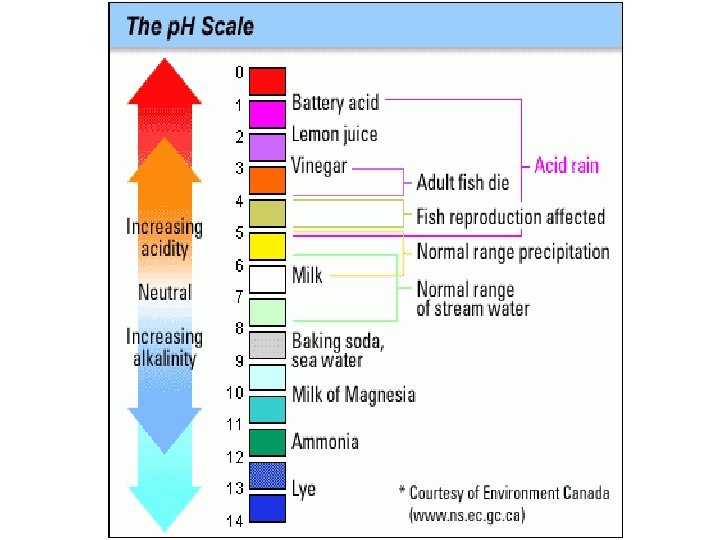
Solubility Will the compound dissolve in water?

Soluble • Refers to whether or not the compound dissolves in water • If it is…. the compound is aqueous (aq) • All acids are soluble • Some ionic compounds are soluble… the rest are solids

Is It soluble? • This will apply to ionic compounds (only) • Steps 1. Find each ion in the boxes across the top 2. if it is soluble it will have (aq) aqueous 3. If it does not dissolve it will have (s) solid.

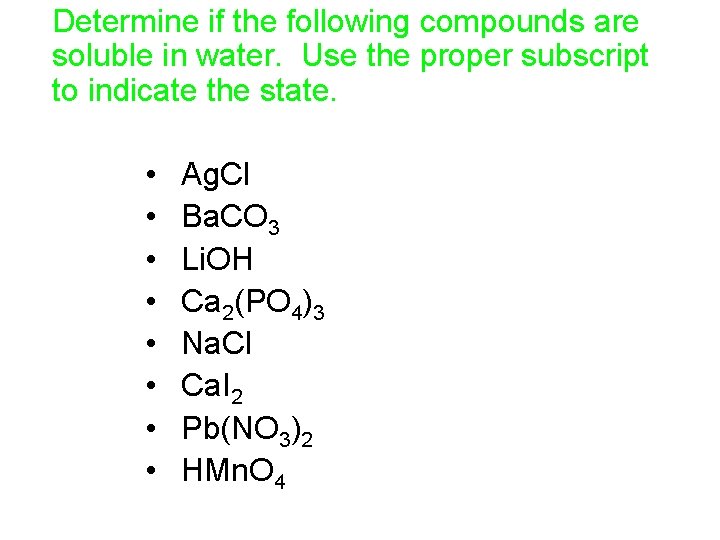
Determine if the following compounds are soluble in water. Use the proper subscript to indicate the state. • • Ag. Cl Ba. CO 3 Li. OH Ca 2(PO 4)3 Na. Cl Ca. I 2 Pb(NO 3)2 HMn. O 4

• Ag. Cl (s) • Ba. CO 3 (s) • Li. OH (aq) • Ca 2(PO 4)3 (s) • Na. Cl (aq) • Ca. I 2 (aq) • Pb(NO 3)2 (aq) • HMn. O 4 (aq)

Acids & Bases


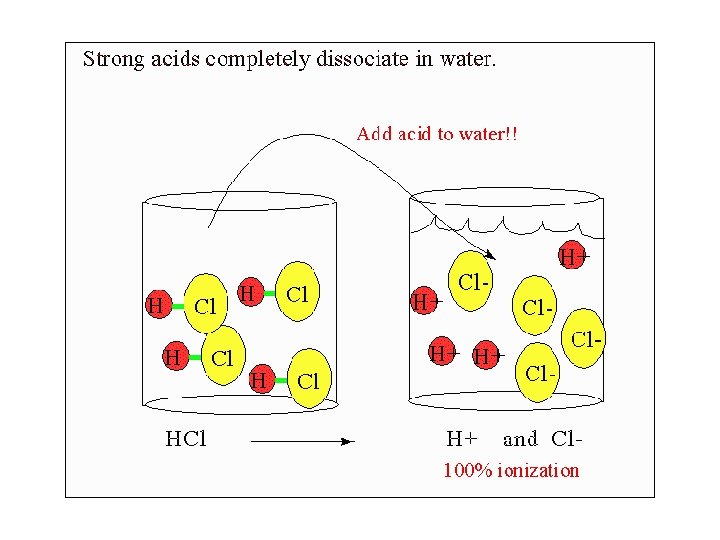
Acids • They are always soluble in water • Conduct electricity • Taste sour • React with metals to produce hydrogen gas (H 2(g)) • Neutralize a base


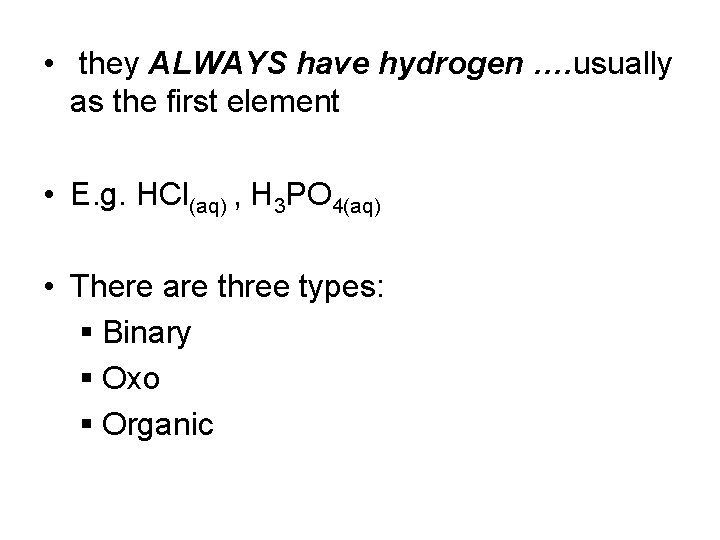
• they ALWAYS have hydrogen …. usually as the first element • E. g. HCl(aq) , H 3 PO 4(aq) • There are three types: § Binary § Oxo § Organic

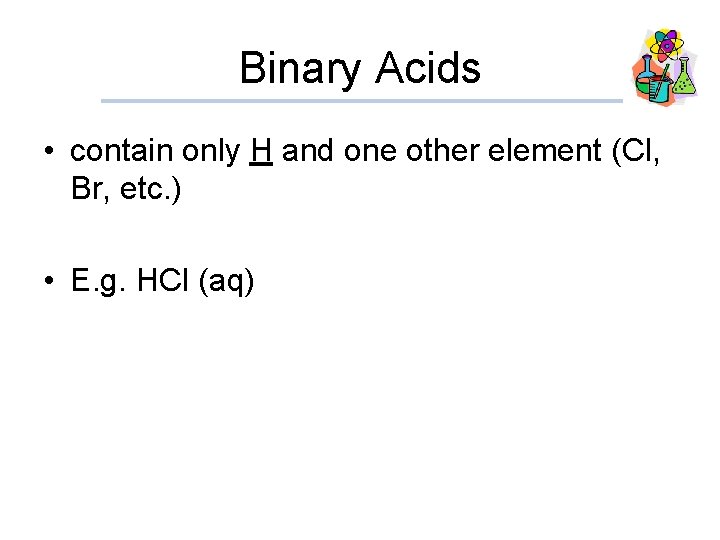
Binary Acids • contain only H and one other element (Cl, Br, etc. ) • E. g. HCl (aq)

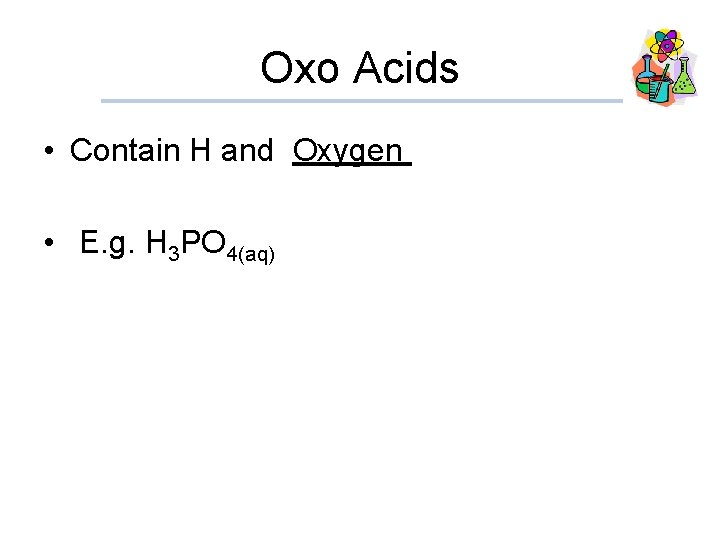
Oxo Acids • Contain H and Oxygen • E. g. H 3 PO 4(aq)

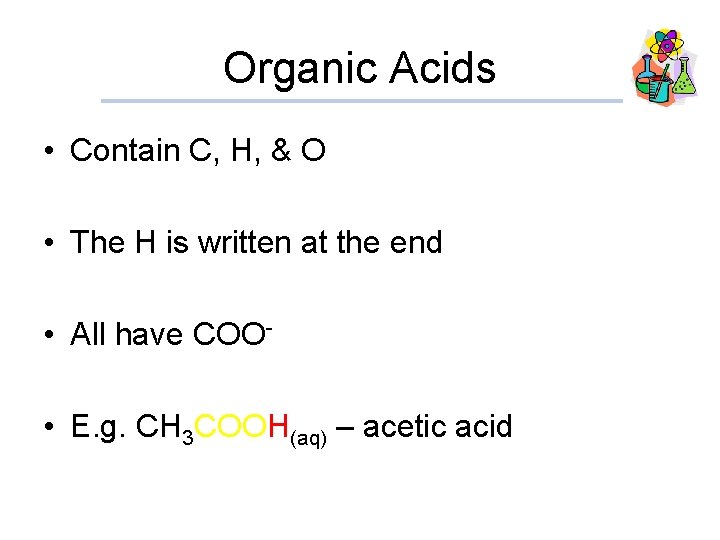
Organic Acids • Contain C, H, & O • The H is written at the end • All have COO • E. g. CH 3 COOH(aq) – acetic acid

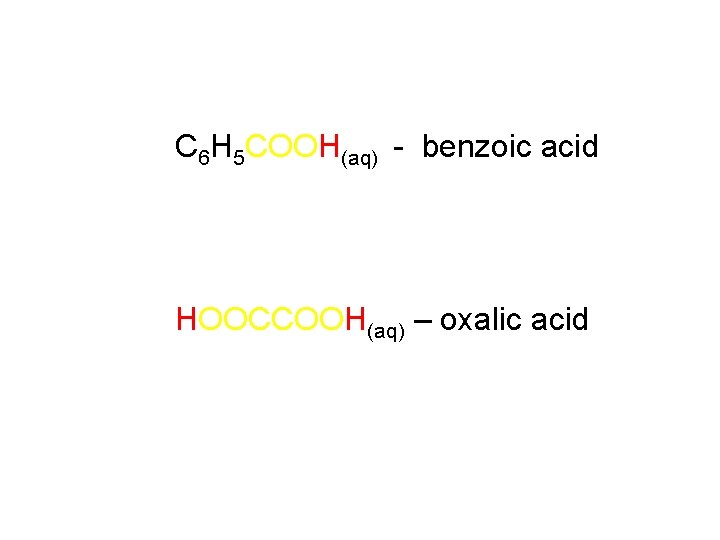
C 6 H 5 COOH(aq) - benzoic acid HOOCCOOH(aq) – oxalic acid

Acid Indicators • Turns blue litmus paper red • Able to turn bromothymol blue to yellow • Phenolphthalein remains colorless • E. g. lemon juice

Bases • Are usually soluble in water • Conduct electricity (not weak ones) • Neutralize acids • Taste bitter • Usually solids • Feel slippery

Base Indicators • Turns red litmus paper blue • Bromothymol blue remains blue • Turns phenolphthalein pink • E. g. baking soda, Rolaids, soap, Draino crystals

Naming Acids • Steps: § Hydrogen ____ide becomes hydro____ic acid § Hydrogen ____ate becomes _______ic acid § Hydrogen ____ ite becomes _______ ous acid

Examples • HF (aq) hydrogen fluoride = hydrofluoric acid • H 2 SO 3 (aq) hydrogen sulphite = sulphurous acid • H 3 BO 3 (aq) hydrogen borate = boric acid Hydrogen chloride (not an acid) • HCl (g)

Try the Following hydrogen sulphide H 2 S (aq) 1+ 2 - hydrosulphuric acid phosphorus acid hydrogen phosphite H 3 PO 3 (aq) 1+ 3 carbonic acid hydrogen carbonate H 2 CO 3(aq) 1+ 2 -

Writing Acid Formulas • Steps: 1. Use the naming rules in the opposite direction • Example: hydrosulphuric acid hydrogen sulphide H 2 S(aq)

Try the Following • carbonic acid hydrogen carbonate H CO 2 3(aq) • chlorous acid hydrogen chlorite HCl. O 2(aq)

Naming Bases • Steps: 1. Write the metal name 1 st 2. Write hydroxide or bicarbonate E. g. Na. OH sodium hydroxide

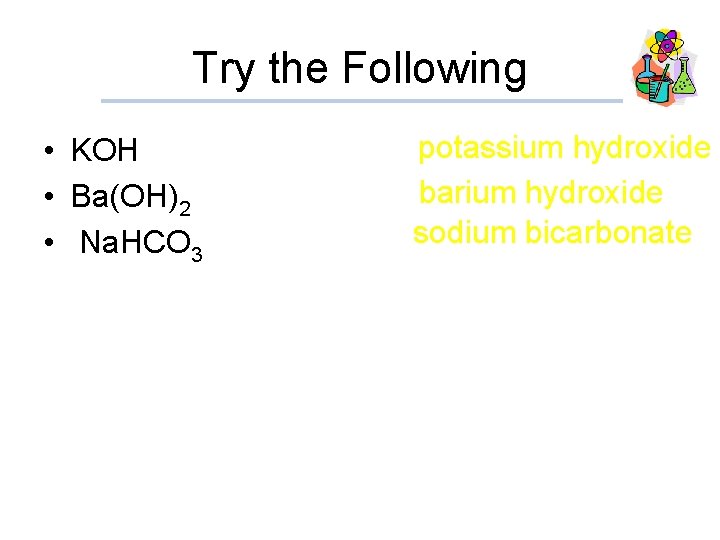
Try the Following • KOH • Ba(OH)2 • Na. HCO 3 potassium hydroxide barium hydroxide sodium bicarbonate

Chemical Reactions • Can cause a physical or a chemical change • Always results in the formation of a new substance • Evidence: 1. Temperature change 2. Formation of a precipitate 3. Colour change 4. Gas produces


Reactants Products balancing 1 Zn(s) + 2 HCl(aq) states + 1 H (g) 1 Zn. Cl 2(aq) 2 states

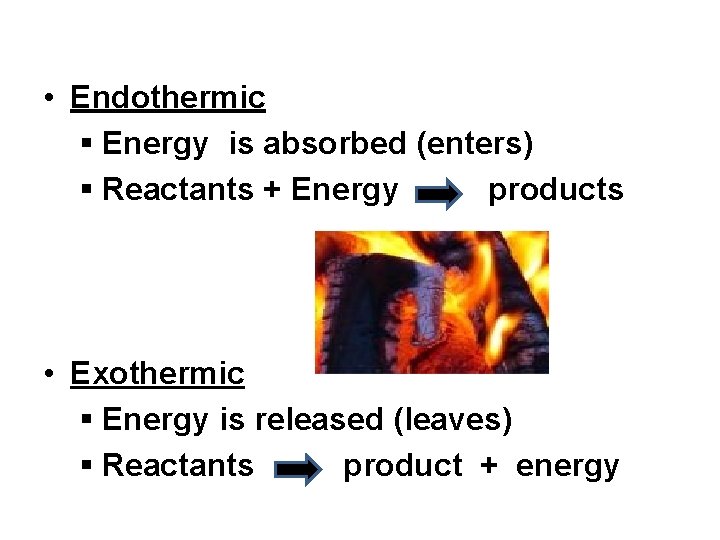
Energy Changes • Can occur in the form of heat, light, electrical, or mechanical • There are two types: § Endothermic § Exothermic

• Endothermic § Energy is absorbed (enters) § Reactants + Energy products • Exothermic § Energy is released (leaves) § Reactants product + energy

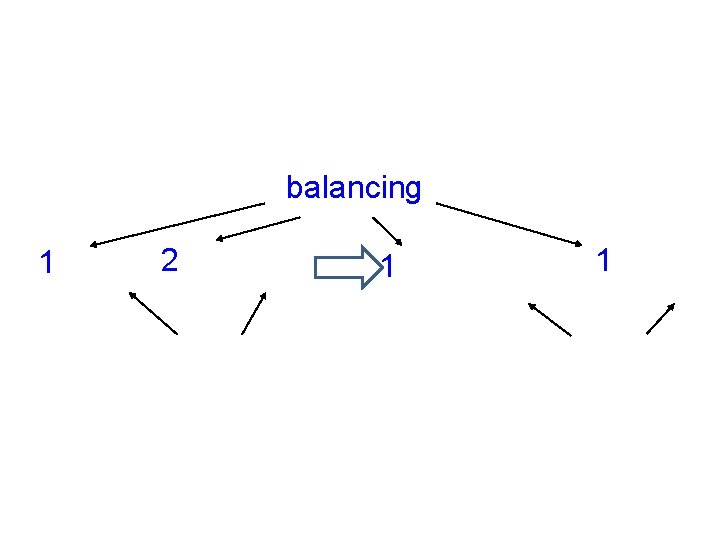
Balancing Equations • There must be equal numbers of each element on both sides of the equation Use lowest numbers

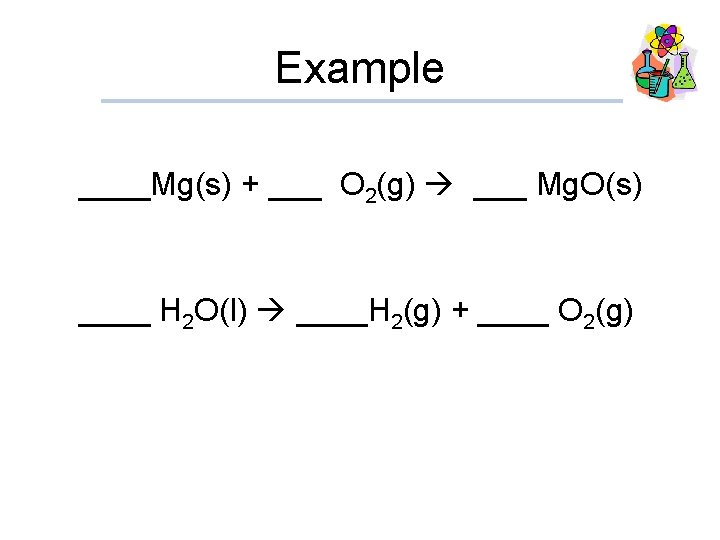
Example ____Mg(s) + ___ O 2(g) ___ Mg. O(s) ____ H 2 O(l) ____H 2(g) + ____ O 2(g)

• When chemicals react they follow the Law of Conservation of Matter: § Matter can not be created or destroyed it only changes form • Mass of reactants = mass of products

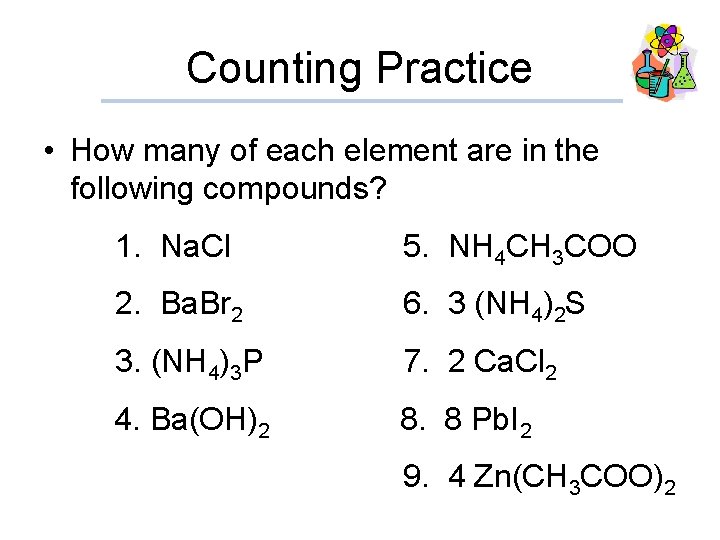
Counting Practice • How many of each element are in the following compounds? 1. Na. Cl 5. NH 4 CH 3 COO 2. Ba. Br 2 6. 3 (NH 4)2 S 3. (NH 4)3 P 7. 2 Ca. Cl 2 4. Ba(OH)2 8. 8 Pb. I 2 9. 4 Zn(CH 3 COO)2

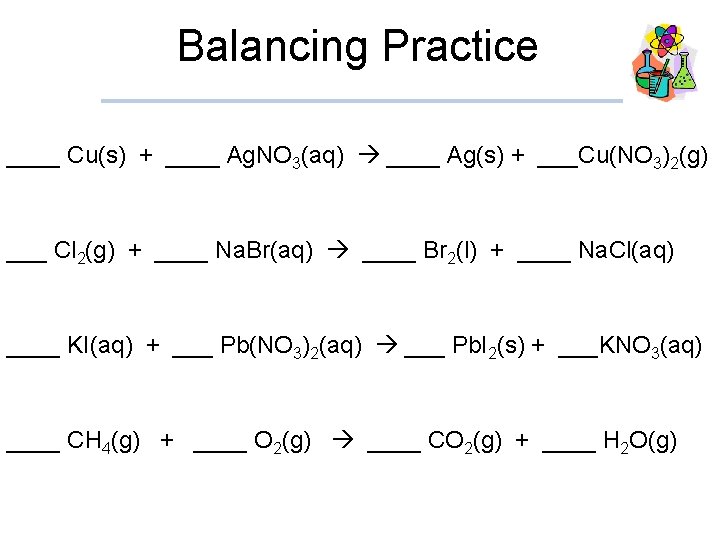
Balancing Practice ____ Cu(s) + ____ Ag. NO 3(aq) ____ Ag(s) + ___Cu(NO 3)2(g) ___ Cl 2(g) + ____ Na. Br(aq) ____ Br 2(l) + ____ Na. Cl(aq) ____ KI(aq) + ___ Pb(NO 3)2(aq) ___ Pb. I 2(s) + ___KNO 3(aq) ____ CH 4(g) + ____ O 2(g) ____ CO 2(g) + ____ H 2 O(g)