Nomenclature A Systematic Approach to Naming Chemical Compounds







- Slides: 19

Nomenclature A Systematic Approach to Naming Chemical Compounds

Nomenclature II Ternary Ionic Compounds Binary and Ternary Acids

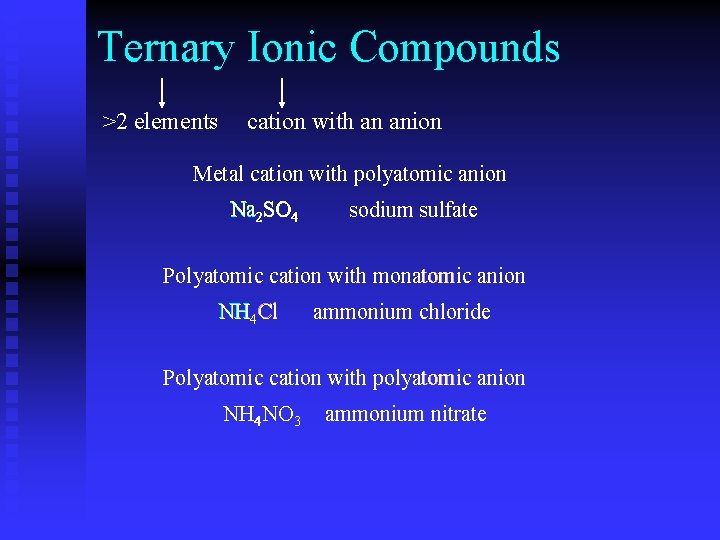
Ternary Ionic Compounds >2 elements cation with an anion Metal cation with polyatomic anion Na 2 SO 4 sodium sulfate Polyatomic cation with monatomic anion NH 4 Cl ammonium chloride Polyatomic cation with polyatomic anion NH 4 NO 3 ammonium nitrate

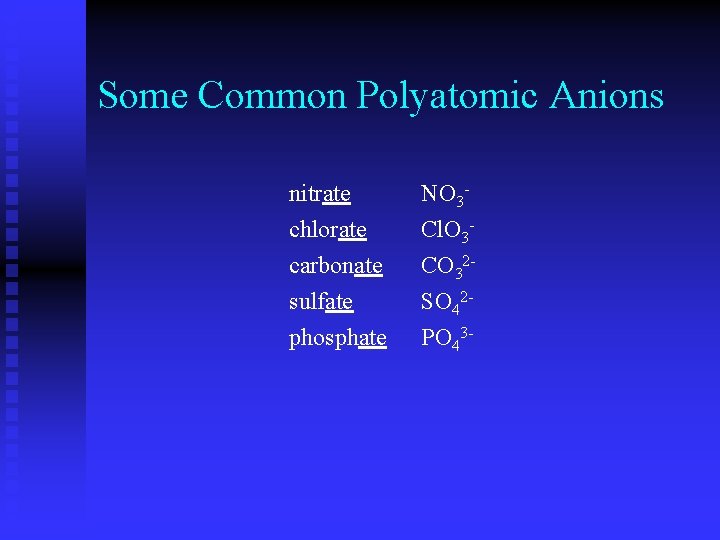
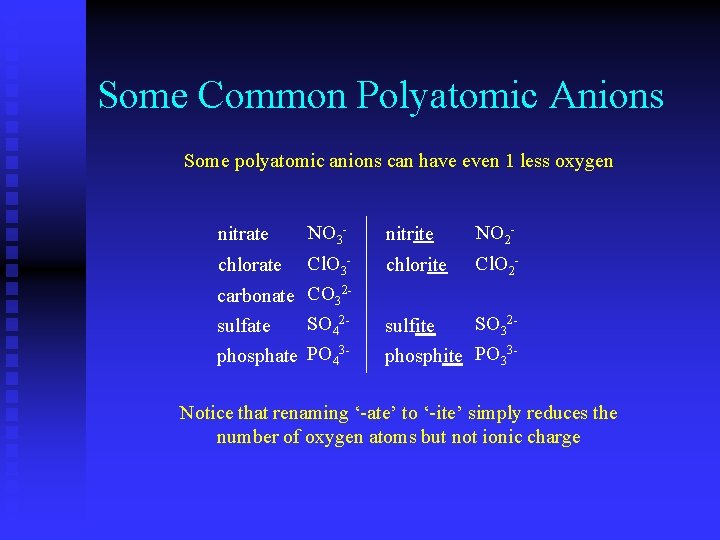
Some Common Polyatomic Anions nitrate chlorate carbonate sulfate phosphate NO 3 Cl. O 3 CO 32 SO 42 PO 43 -

Some Common Polyatomic Anions nitrate NO 3 nitrate chlorate Cl. O 3 chlorate carbonate CO 32 carbonatesulfate SO 42 sulfate phosphate 3 phosphate PO 4 NO 3 Cl. O 3 CO 32 SO 42 PO 43 -

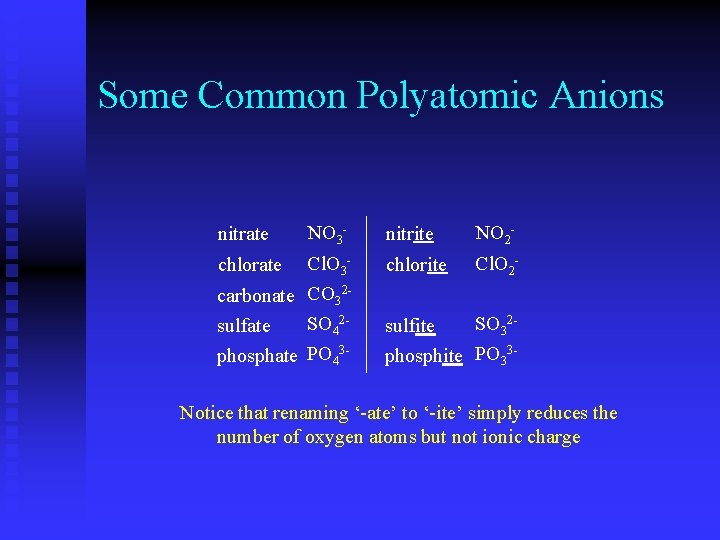
Some Common Polyatomic Anions nitrate NO 3 - nitrite NO 2 - chlorate Cl. O 3 - chlorite Cl. O 2 - carbonate CO 32 SO 42 sulfate phosphate PO 43 - SO 32 sulfite phosphite PO 33 - Notice that renaming ‘-ate’ to ‘-ite’ simply reduces the number of oxygen atoms but not ionic charge

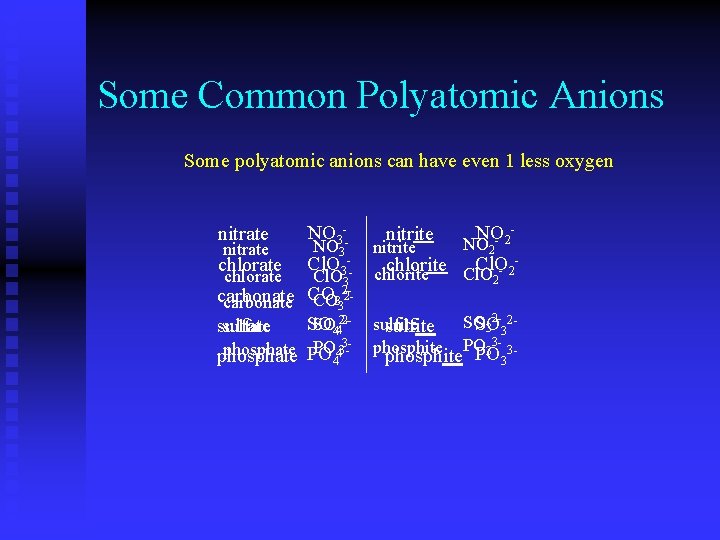
Some Common Polyatomic Anions Some polyatomic anions can have even 1 less oxygen nitrate NO 3 - nitrite NO 2 - chlorate Cl. O 3 - chlorite Cl. O 2 - carbonate CO 32 SO 42 sulfate phosphate PO 43 - SO 32 sulfite phosphite PO 33 - Notice that renaming ‘-ate’ to ‘-ite’ simply reduces the number of oxygen atoms but not ionic charge

Some Common Polyatomic Anions Some polyatomic anions can have even 1 less oxygen nitrate NO 3 -NO 3 nitrite NO- 2 - NO 2 Cl. O 3 -- chlorite Cl. O- 2 chlorite Cl. O 3 chlorate 2 2 carbonate CO 332 carbonate CO 2 - 2 SO 442 -2 - sulfite SO SO sulfate 3 3 sulfite SO sulfate 3 PO 33 - 3 PO phosphite 3 phosphate 4 phosphate PO 4 phosphite PO 3 chlorate

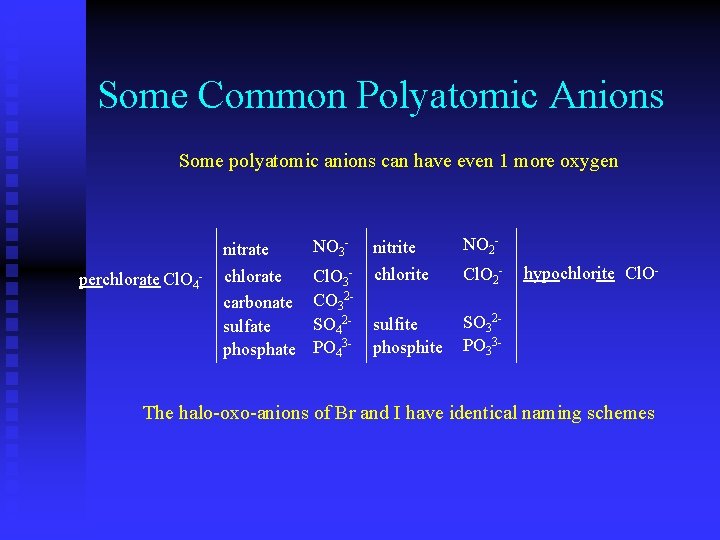
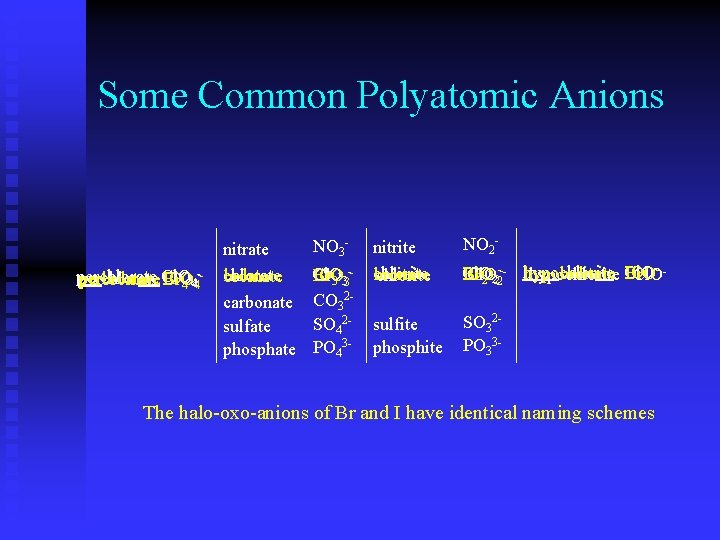
Some Common Polyatomic Anions Some polyatomic anions can have even 1 more oxygen perchlorate Cl. O 4 - nitrate NO 3 - nitrite NO 2 - chlorate carbonate sulfate phosphate Cl. O 3 CO 32 SO 42 PO 43 - chlorite Cl. O 2 - sulfite phosphite SO 32 PO 33 - hypochlorite Cl. O- The halo-oxo-anions of Br and I have identical naming schemes

Some Common Polyatomic Anions perchlorate periodate Cl. O IO 4 -44 -perbromate Br. O nitrate chlorate iodate bromate carbonate sulfate phosphate NO 3 - -Cl. O IO Cl. O Br. O 3 33 CO 32 SO 42 PO 43 - NO 2 - nitrite chlorite iodite bromite chlorite - --- hypochlorite IO Cl. O hypoiodite Br. O IO hypobromite Br. O Cl. O hypochlorite Cl. O 2 222 sulfite phosphite SO 32 PO 33 - The halo-oxo-anions of Br and I have identical naming schemes

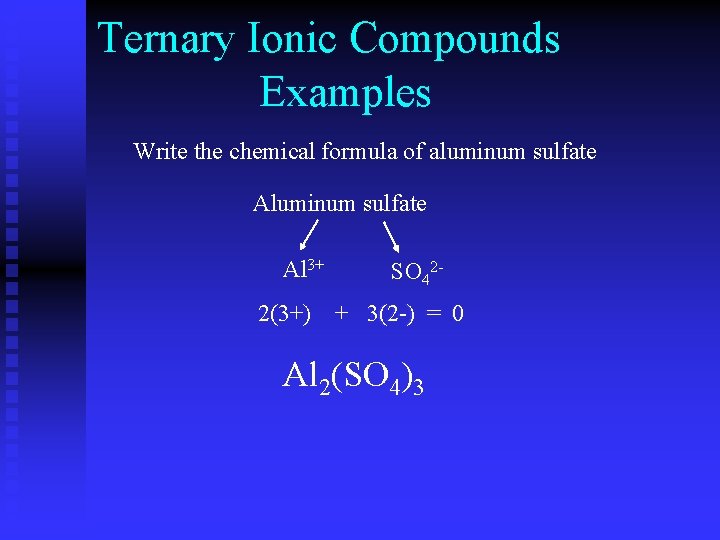
Ternary Ionic Compounds Examples Write the chemical formula of aluminum sulfate Al 3+ SO 42 - 2(3+) ? (3+) + 3(2 -) ? (2 -) = 0 Al 2(SO 4)3

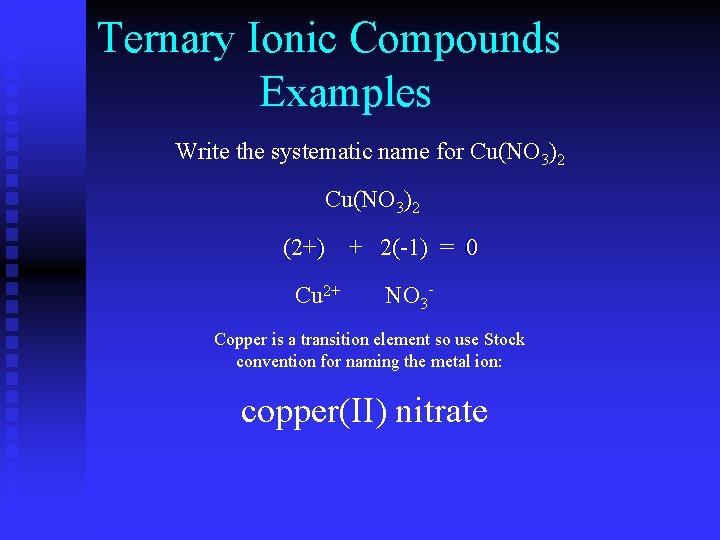
Ternary Ionic Compounds Examples Write the systematic name for Cu(NO 3)2 (? +) + 2(-1) = 0 (2+) ? + Cu 2+ NO 3 - Copper is a transition element so use Stock convention for naming the metal ion: copper(II) nitrate

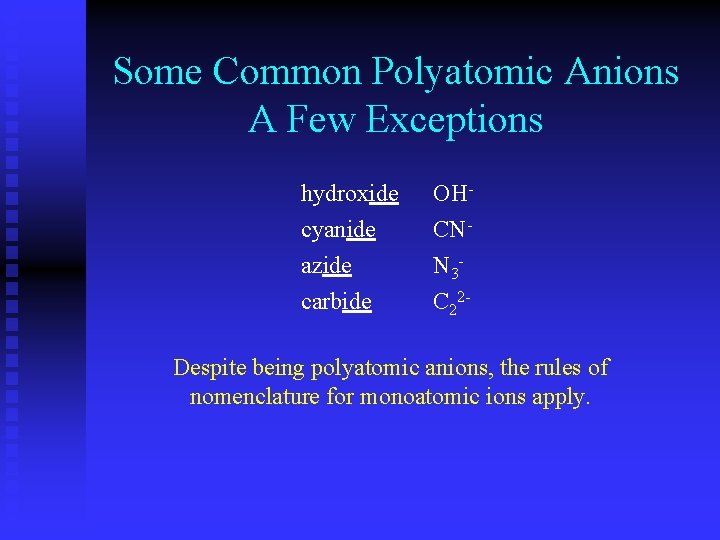
Some Common Polyatomic Anions A Few Exceptions hydroxide cyanide azide carbide OHCNN 3 C 22 - Despite being polyatomic anions, the rules of nomenclature for monoatomic ions apply.

Binary and Ternary Acids A binary acid is molecular compound which dissociates into hydrogen ion and a monoatomic anion in aqueous solution. A ternary acid is molecular compound which is a combination of hydrogen ion and a polyatomic anion.

Binary Acids Prefix the root name of the anion with ‘hydro’ Transform the suffix of the anion from ‘-ide’ to ‘-ic’ Add the word ‘acid’ EXAMPLES HCl(g) hydrogen Read the formula chloridecarefully! HCl(aq) hydrochloric acid HI(g) hydrogen iodide HI(aq) hydroiodic acid

Binary Acids An Exception HCN(g) hydrogen cyanide HCN(aq) hydrocyanic Read the molecular acid name carefully Even though it is not a binary acid, the ‘-ide’ ending of the anion requires application of the binary acid rule.

Ternary Acids Transform the suffix of the anion from ‘-ate’ to ‘-ic’ -or. Transform the suffix of the anion from ‘-ite’ to ‘-ous’ Add the word ‘acid’ Never use the prefix ‘hydro’ for ternary acids EXAMPLES HNO 3 nitric acid HNO 2 nitrous acid

Ternary Acids The name does not include ‘acid’ until the anion’s ionic charge is completely balanced by hydrogen ion. EXAMPLE Na 2 SO 4 sodium sulfate Na. HSO 4 sodium hydrogen sulfate H 2 SO 4 sulfuric acid

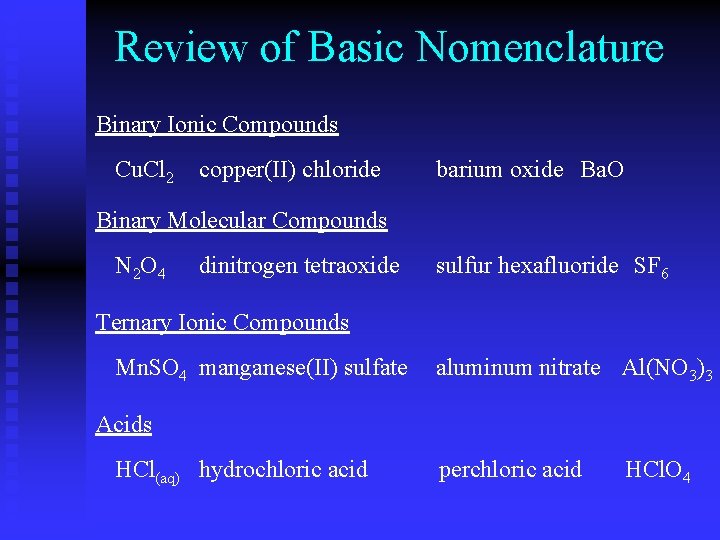
Review of Basic Nomenclature Binary Ionic Compounds Cu. Cl 2 copper(II) chloride barium oxide Ba. O Binary Molecular Compounds N 2 O 4 dinitrogen tetraoxide sulfur hexafluoride SF 6 Ternary Ionic Compounds Mn. SO 4 manganese(II) sulfate aluminum nitrate Al(NO 3)3 Acids HCl(aq) hydrochloric acid perchloric acid HCl. O 4