9 4 Naming and Writing Formulas for Acids



































- Slides: 50

9. 4 Naming and Writing Formulas for Acids and Bases > Chapter 9 Chemical Names and Formulas 9. 1 Naming Ions 9. 2 Naming and Writing Formulas for Ionic Compounds 9. 3 Naming and Writing Formulas for Molecular Compounds 9. 4 Naming and Writing Formulas for Acids and Bases 9. 5 The Laws Governing How Compounds Form 1 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > CHEMISTRY & YOU What’s the name of the acid responsible for the crisp taste in this drink? There’s a certain acid that gives many soft drinks their crisp, enjoyable taste. 2 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids How do you determine the name and formula of an acid? 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids • Acids are a group of ionic compounds with unique properties. • Acids can be defined in several ways. • For now, it is enough to know that an acid is a compound that contains one or more hydrogen atoms and produces hydrogen ions when dissolved in water. 4 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

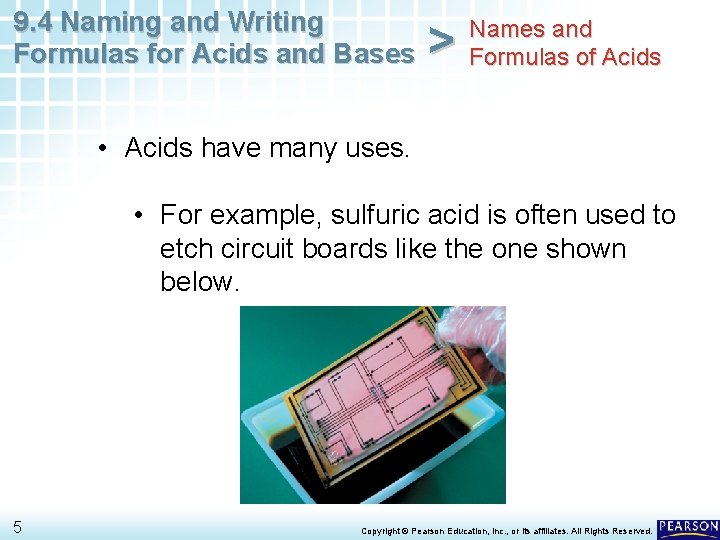
9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids • Acids have many uses. • For example, sulfuric acid is often used to etch circuit boards like the one shown below. 5 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids • When naming an acid, you can consider the acid to consist of an anion combined with as many hydrogen ions as needed to make the molecule electrically neutral. • Therefore, the chemical formulas of acids are in the general form Hn. X, where X is a monatomic or polyatomic anion and n is a subscript indicating the number of hydrogen ions that are combined with the anion. 6 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

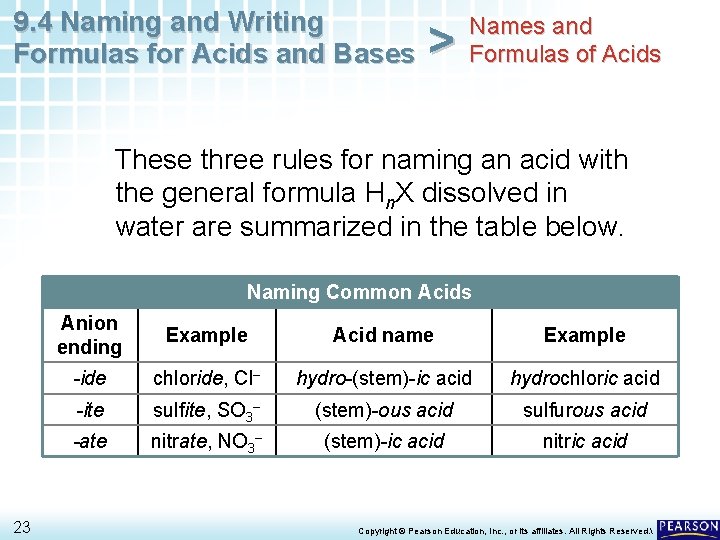
9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids • Three rules can help you name an acid with the general formula Hn. X dissolved in water. • The naming system depends on the name of the anion (X), in particular the suffix of the anion name. • Each rule deals with an anion with a different suffix: -ide, -ite, and -ate. 7 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids • Three rules can help you name an acid with the general formula Hn. X dissolved in water. 1. When the name of the anion ends in ide, the acid name begins with the prefix hydro-. The stem of the anion has the suffix -ic and is followed by the word acid. These acids are known as Binary Acids • Therefore, HCl (X= chloride) is named hydrochloric acid. 8 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

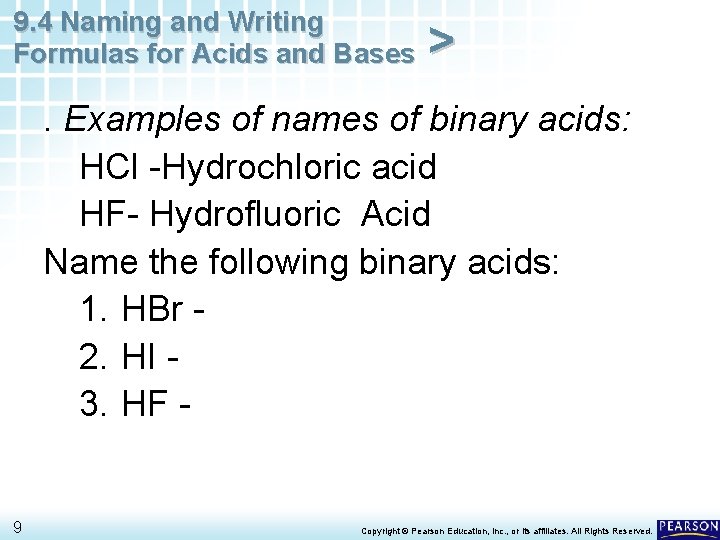
9. 4 Naming and Writing Formulas for Acids and Bases > . Examples of names of binary acids: HCl -Hydrochloric acid HF- Hydrofluoric Acid Name the following binary acids: 1. HBr 2. HI 3. HF - 9 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

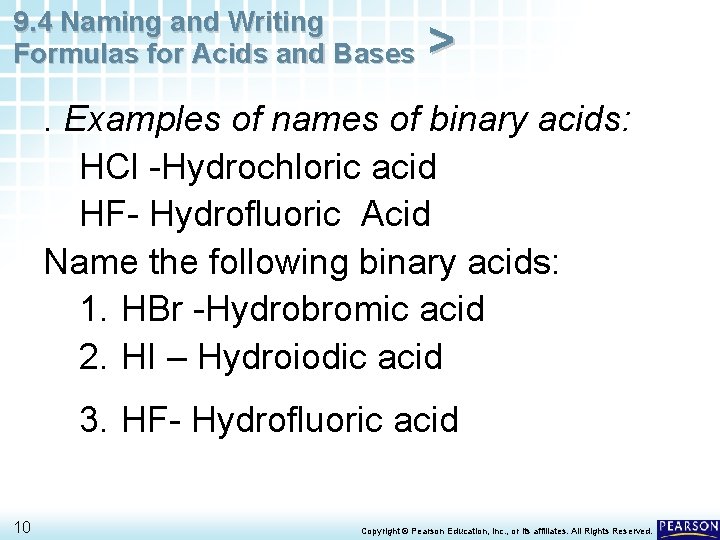
9. 4 Naming and Writing Formulas for Acids and Bases > . Examples of names of binary acids: HCl -Hydrochloric acid HF- Hydrofluoric Acid Name the following binary acids: 1. HBr -Hydrobromic acid 2. HI – Hydroiodic acid 3. HF- Hydrofluoric acid 10 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids • Three rules can help you name an acid with the general formula Hn. X dissolved in water. 2. When the anion name ends in -ite, the acid name is the stem of the anion with the suffix -ous, followed by the word acid. • Thus, H 2 SO 3 (X = sulfite) is named sulfurous acid. 11 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

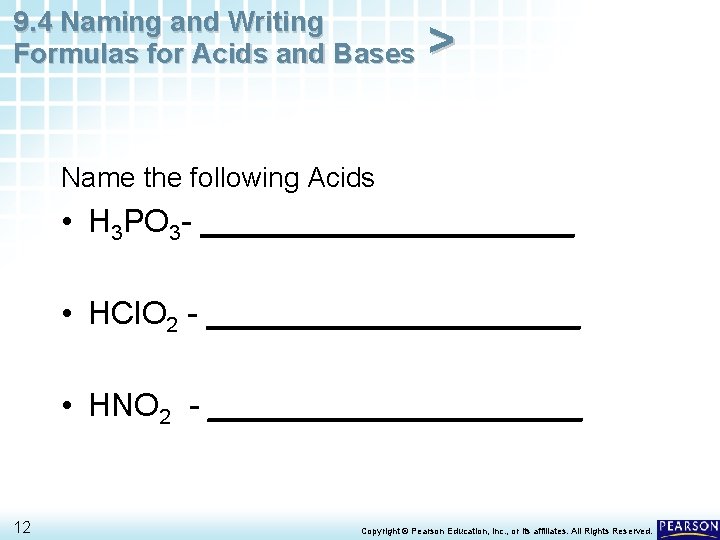
9. 4 Naming and Writing Formulas for Acids and Bases > Name the following Acids • H 3 PO 3 - ___________ • HCl. O 2 - ___________ • HNO 2 - ___________ 12 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Name the following Acids • H 3 PO 3 - Phosphorous Acid • • HCl. O 2 - Chlorous acid • HNO 2 - Nitrous Acid 13 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids • Three rules can help you name an acid with the general formula Hn. X dissolved in water. 3. When the anion name ends in -ate, the acid name is the stem of the anion with the suffix -ic, followed by the word acid. • Thus, HNO 3 (X = nitrate) is named nitric acid. 14 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

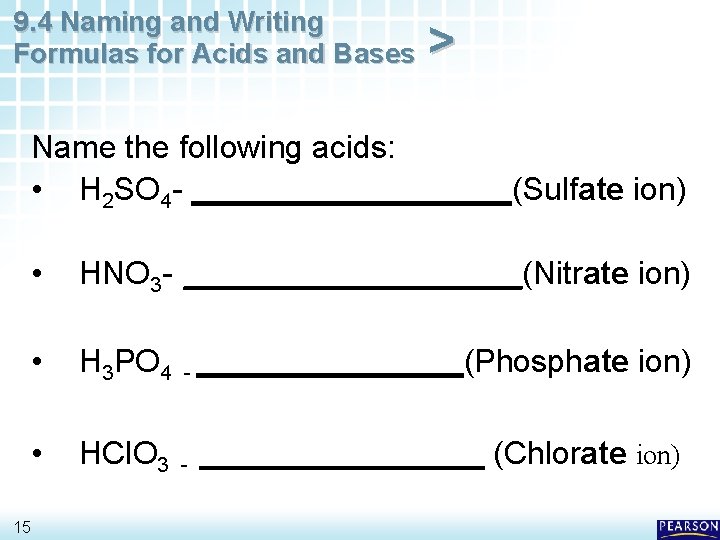
9. 4 Naming and Writing Formulas for Acids and Bases > Name the following acids: • H 2 SO 4 - _________(Sulfate ion) 15 • HNO 3 - __________(Nitrate ion) • H 3 PO 4 - ________(Phosphate ion) • HCl. O 3 - ________ (Chlorate ion)

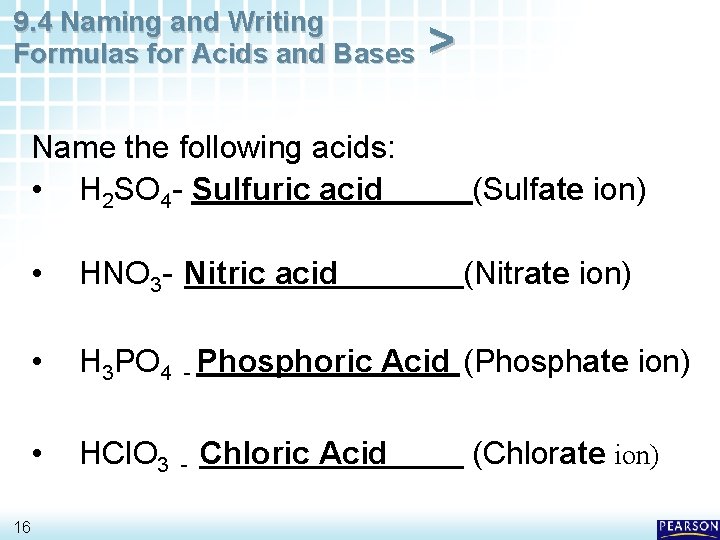
9. 4 Naming and Writing Formulas for Acids and Bases 16 > Name the following acids: • H 2 SO 4 - Sulfuric acid (Sulfate ion) • HNO 3 - Nitric acid (Nitrate ion) • H 3 PO 4 - Phosphoric Acid (Phosphate ion) • HCl. O 3 - Chloric Acid (Chlorate ion)

9. 4 Naming and Writing Formulas for Acids and Bases Acids > For the acid containing the polyatomic ion with two less oxygens than the -ic(-ate ion), use the prefix hypo and the suffix ous. (element name) + ous acid Examples: H 2 SO 2 - hyposulfurous acid HNO - hyponitrous acid 17

9. 4 Naming and Writing Formulas for Acids and Bases Acids > For an acid containing the polyatomic with one more oxygen than the ic (-ate ion), use the prefix per and the suffix ic. Examples: HCl. O 4 - perchloric acid (chlorate radical Cl. O 3 + 1 oxygen ) HBr. O 4 - perbromic acid (bromate radical Br. O 3 + 1 oxygen) 18

9. 4 Naming and Writing Formulas for Acids and Bases Acids Name the following acids: 1) H 3 PO 4 2) H 2 CO 3 3) H 2 SO 4 4) HIO 3 5) HF 6) HNO 2 19 >

9. 4 Naming and Writing Formulas for Acids and Bases Acids > Name the following acids: 1. H 3 PO 4 - - phosphoric acid 2. H 2 CO 3 - carbonic acid 3. H 2 SO 4 - sulfuric acid 4. HIO 3 - iodic acid 5. HF - hydrofluoric acid 6. HNO 2 - nitrous acid 20

9. 4 Naming and Writing Formulas for Acids and Bases Acids > Write the correct name these four acids involving chlorine. The answers and an explanation for each name follows 1. HCl 2. HCl. O 3. HCl. O 2 4. HCl. O 3 5. HCl. O 4 21

9. 4 Naming and Writing Formulas for Acids and Bases Acids > Write the correct name these four acids involving chlorine. The answers and an explanation for each name follows 1. 2. 3. 4. 5. 22 HCl - Hydrochloric Acid HCl. O - Hypochlorous Acid HCl. O 2 - Chlorous Acid HCl. O 3 - Chloric Acid` HCl. O 4 - Perchloric Acid

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids These three rules for naming an acid with the general formula Hn. X dissolved in water are summarized in the table below. Naming Common Acids 23 Anion ending Example Acid name Example -ide chloride, Cl– hydro-(stem)-ic acid hydrochloric acid -ite sulfite, SO 3– (stem)-ous acid sulfurous acid -ate nitrate, NO 3– (stem)-ic acid nitric acid Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > CHEMISTRY & YOU An acid that provides the crisp taste in many soft drinks has the formula H 3 PO 4. What’s the name of this acid? 24 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > CHEMISTRY & YOU An acid that provides the crisp taste in many soft drinks has the formula H 3 PO 4. What’s the name of this acid? The name of this acid is phosphoric acid. 25 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids Writing Formulas of Acids To write the formula for an acid, use the rule for writing the name of the acid in reverse. Then, balance the ionic charges just as you would for any ionic compound. 26 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids Writing Formulas of Acids • For example, consider hydrobromic acid. • Rule 1 states: When the name of the anion ends in -ide, the acid name begins with the prefix hydro-. The stem of the anion has the suffix -ic and is followed by the word acid. • Following Rule 1, hydrobromic acid (hydroprefix and -ic suffix) must be a combination of hydrogen ion (H+) and bromide ion (Br –). • 27 The formula is HBr. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids Writing Formulas of Acids • How do you write the formula for phosphorous acid? • Rule 2 states: When the anion name ends in -ite, the acid name is the stem of the anion with the suffix -ous, followed by the word acid. • Using Rule 1, hydrogen ion and phosphite ion (PO 33–) must be the components of phosphorous acid. • You need three hydrogen ions to balance the 3– charge of the phosphite ion. • Thus, the formula for phosphorous acid is H 3 PO 3. 28 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids Writing Formulas of Acids • Finally, what is the formula for sulfuric acid? • Rule 3 states: When the anion name ends in -ate, the acid name is the stem of the anion with the suffix -ic, followed by the word acid. • According to Rule 3, sulfuric acid (-ic ending) must be a combination of hydrogen ion and sulfate ion (SO 42–). • The formula for sulfuric acid is H 2 SO 4 because two hydrogen ions are needed to balance the 2– charge of the sulfate anion. 29 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases Acids > Write the formula for these acids: 7. hydrobromic acid -__________ 8. hydrocyanic acid - _________ 9. nitric acid - ___________ 10. sulfurous acid - _________ 11. phosphorous acid – ________ 12. acetic acid – __________ 30

9. 4 Naming and Writing Formulas for Acids and Bases Acids > Write the formula for these acids: 7. hydrobromic acid -HBr 8. hydrocyanic acid - HCN 9. nitric acid - HNO 3 10. sulfurous acid _ H 2 SO 3 11. phosphorous acid – H 3 PO 3 12. acetic acid – HC 2 H 3 O 2 31

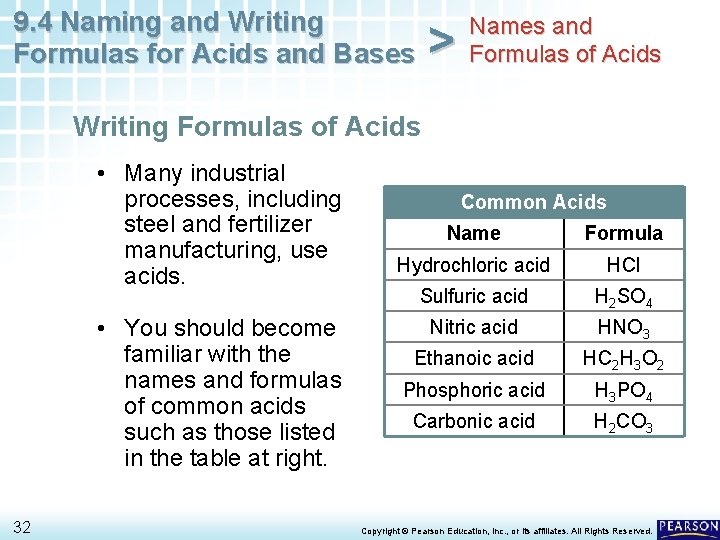
9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Acids Writing Formulas of Acids • Many industrial processes, including steel and fertilizer manufacturing, use acids. • You should become familiar with the names and formulas of common acids such as those listed in the table at right. 32 Common Acids Name Formula Hydrochloric acid HCl Sulfuric acid H 2 SO 4 Nitric acid HNO 3 Ethanoic acid HC 2 H 3 O 2 Phosphoric acid H 3 PO 4 Carbonic acid H 2 CO 3 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

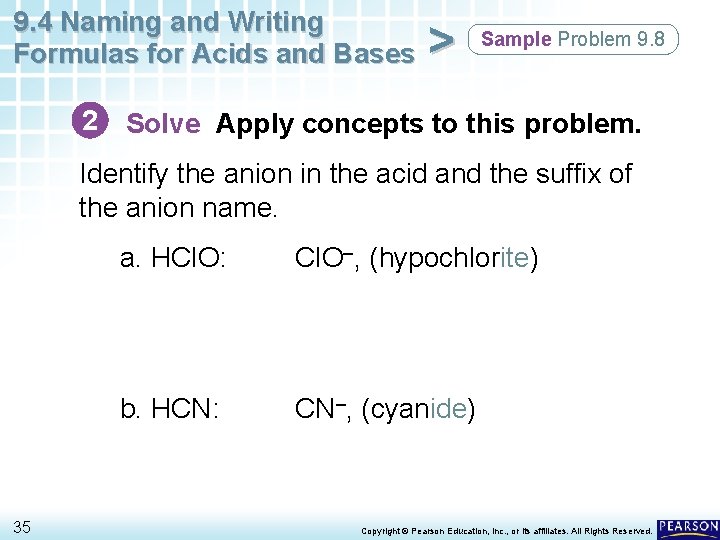
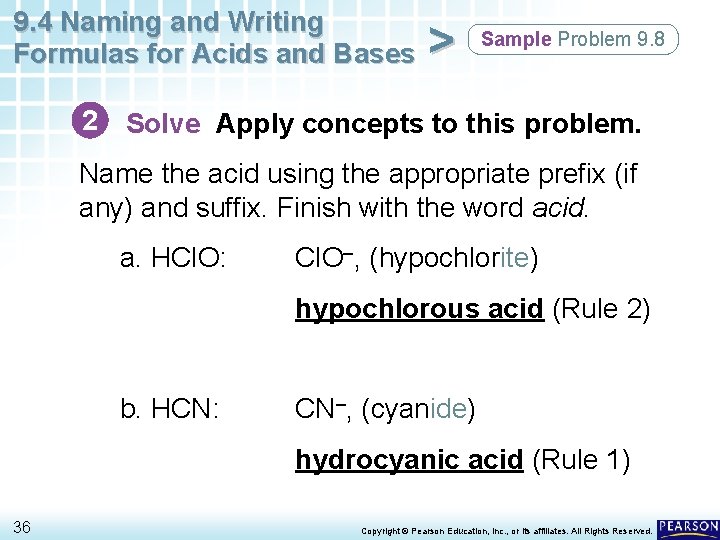
9. 4 Naming and Writing Formulas for Acids and Bases > Sample Problem 9. 8 Naming Acids Name the following compounds as acids. a. HCl. O b. HCN 33 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Sample Problem 9. 8 Formulas for Acids and Bases Analyze Identify the relevant concepts. > 1 The anion of the acid determines the acid name. (1) If the name of the anion ends in -ide, name the acid using the stem of the anion with the prefix hydro- and the suffix -ic, followed by the word acid. (2) If the anion name ends in -ite, name the acid using the stem of the anion with the suffix -ous, followed by the word acid. 34 (3) If the anion name ends in -ate, name the acid using the stem of the anion with the suffix -ic, followed by the word acid. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Sample Problem 9. 8 2 Solve Apply concepts to this problem. Identify the anion in the acid and the suffix of the anion name. 35 a. HCl. O: Cl. O–, (hypochlorite) b. HCN: CN–, (cyanide) Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Sample Problem 9. 8 2 Solve Apply concepts to this problem. Name the acid using the appropriate prefix (if any) and suffix. Finish with the word acid. a. HCl. O: Cl. O–, (hypochlorite) hypochlorous acid (Rule 2) b. HCN: CN–, (cyanide) hydrocyanic acid (Rule 1) 36 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > What is the name of the acid H 2 S? (Hint: X = sulfide) 37 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > What is the name of the acid H 2 S? (Hint: X = sulfide) H 2 S is named hydrosulfuric acid. Use Rule 1: When the name of the anion ends in -ide, the acid name begins with the prefix hydro-. The stem of the anion has the suffix -ic and is followed by the word acid. 38 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Bases How do you determine the name and formula of a base? 39 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Bases • A base is generally an ionic compound that produces hydroxide ions when dissolved in water. Bases are named in the same way as other ionic compounds—the name of the cation is followed by the name of the anion. 40 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Bases • The common base sodium hydroxide is used in making cleaners, soap, and paper, as shown in the figure at right. • Sodium hydroxide (Na. OH) is composed of sodium cations (Na+) and hydroxide anions (OH–). 41 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Bases To write the formula for a base, 1. first write the symbol for the metal cation 2. followed by the formula for the hydroxide ion. 3. Then, balance the ionic charges just as you would for any ionic compound. 42 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Names and Formulas of Bases • For example, aluminum hydroxide consists of the aluminum cation (Al 3+) and the hydroxide anion (OH–). • You need three hydroxide ions to balance the 3+ charge of the aluminum cation. • Thus, the formula for aluminum hydroxide is Al(OH)3. 43 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

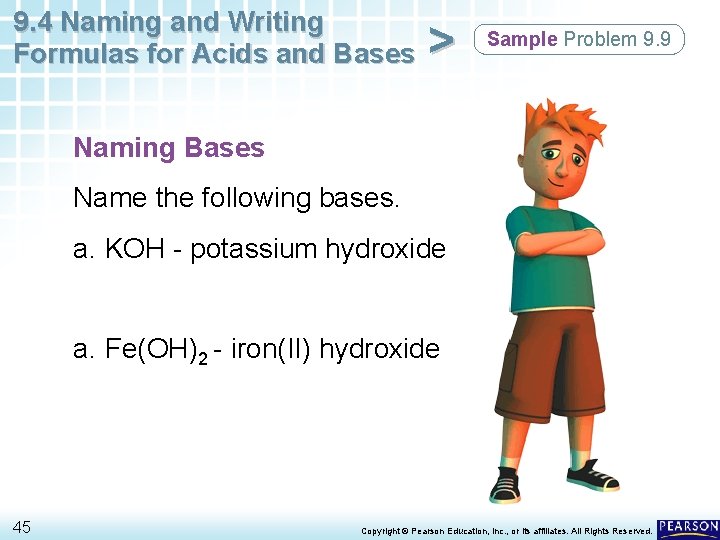
9. 4 Naming and Writing Formulas for Acids and Bases > Sample Problem 9. 9 Naming Bases Name the following bases. a. KOH a. Fe(OH)2 44 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Sample Problem 9. 9 Naming Bases Name the following bases. a. KOH - potassium hydroxide a. Fe(OH)2 - iron(II) hydroxide 45 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Is the naming of a base more similar to the naming of an acid or to the naming of other ionic compounds? Unlike acids, bases are named in the same way as other ionic compounds. 46 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Key Concepts 1. 47 If the anion name ends in -ide, the acid name begins with the prefix hydro-. The stem of the anion has the suffix -ic and is followed by the word acid. 2. If the anion name ends in -ite, the acid name is the stem of the anion with the suffix -ous, followed by the word acid. 3. If the anion name ends in -ate, the acid name is the stem of the anion with the suffix -ic, followed by the word acid. 4. To write the formula for an acid, use the rule for writing the name of the acid in reverse. Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

9. 4 Naming and Writing Formulas for Acids and Bases > Key Concepts Bases are named like other ionic compounds. To write the formula for a base, write the symbol for the metal cation followed by that of the hydroxide ion. Then, balance the ionic charges. 48 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

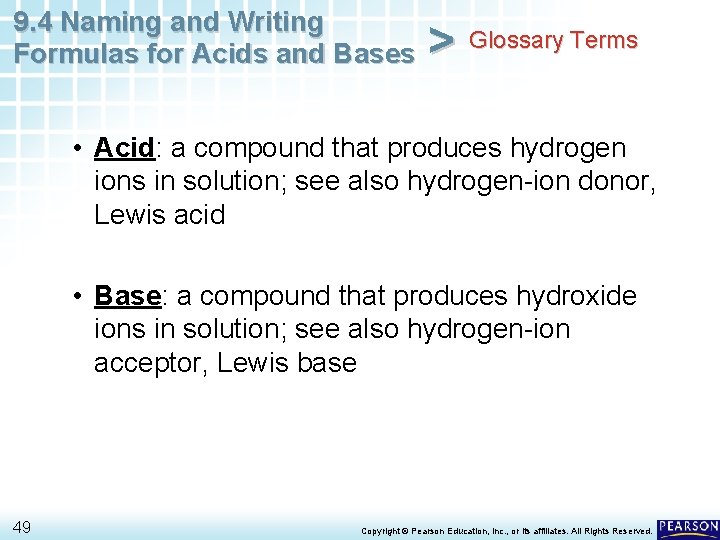
9. 4 Naming and Writing Formulas for Acids and Bases > Glossary Terms • Acid: a compound that produces hydrogen ions in solution; see also hydrogen-ion donor, Lewis acid • Base: a compound that produces hydroxide ions in solution; see also hydrogen-ion acceptor, Lewis base 49 Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.

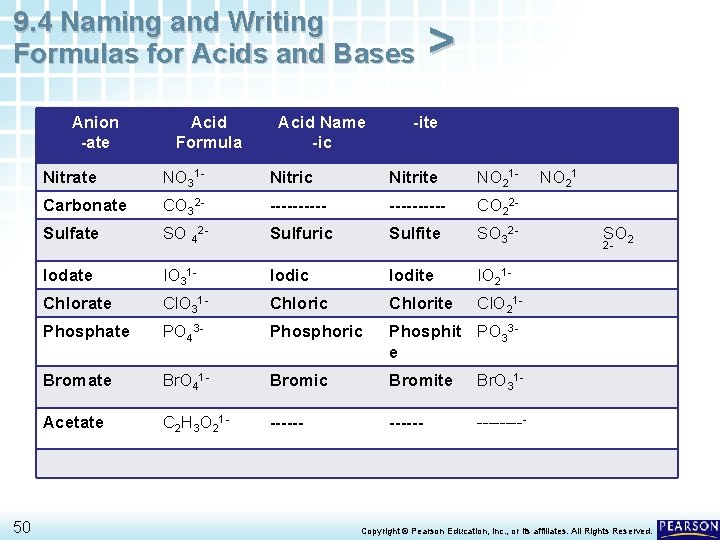
9. 4 Naming and Writing Formulas for Acids and Bases Anion -ate 50 Acid Formula Acid Name -ic > -ite Nitrate NO 31 - Nitric Nitrite NO 21 - Carbonate CO 32 - ---------- CO 22 - Sulfate SO 42 - Sulfuric Sulfite SO 32 - Iodate IO 31 - Iodic Iodite IO 21 - Chlorate Cl. O 31 - Chloric Chlorite Cl. O 21 - Phosphate PO 43 - Phosphoric Phosphit PO 33 e Bromate Br. O 41 - Bromic Bromite Br. O 31 - Acetate C 2 H 3 O 21 - ------ NO 21 SO 2 2 - Copyright © Pearson Education, Inc. , or its affiliates. All Rights Reserved.