Naming Compounds and Writing Formulas for Compounds Systematic

















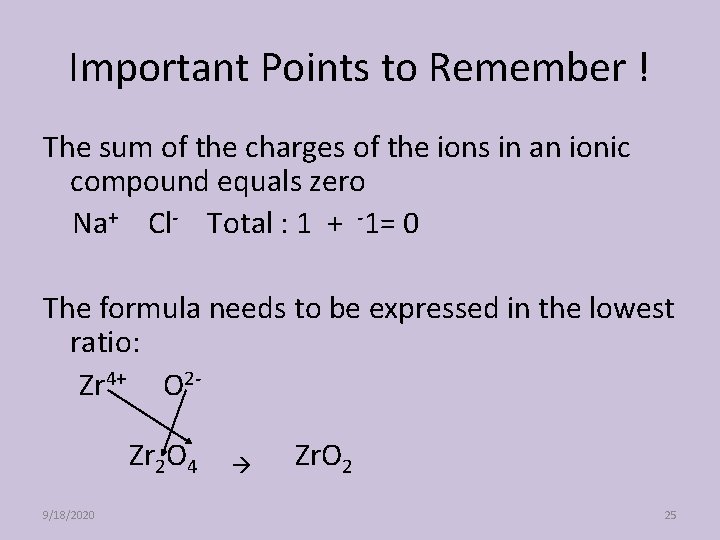




- Slides: 32

Naming Compounds and Writing Formulas for Compounds

Systematic Naming of Compounds STOCK SYSTEM OF NAMING • There are too many compounds to remember the names of them all. • A compound is made of two or more elements chemically combined. • Name should tell us how many and what type of atoms.

Two Types of Compounds 1. Ionic Compounds • Made of cations and anions. • Metals and nonmetals. • The electrons lost by the cation are gained by the anion. • The cation and anions surround each other. • Smallest piece is a FORMULA UNIT.

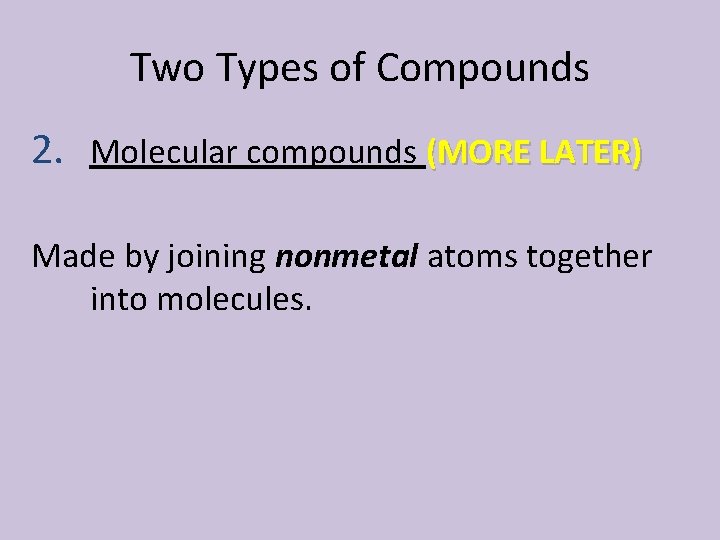
Two Types of Compounds 2. Molecular compounds (MORE LATER) Made by joining nonmetal atoms together into molecules.

REVIEW: Atoms and ions • Atoms are electrically neutral. • Same number of protons and electrons. • Ions are atoms, or groups of atoms, with a charge (positive or negative) • Different numbers of protons and electrons. • Only electrons can move. • Gain or lose electrons.

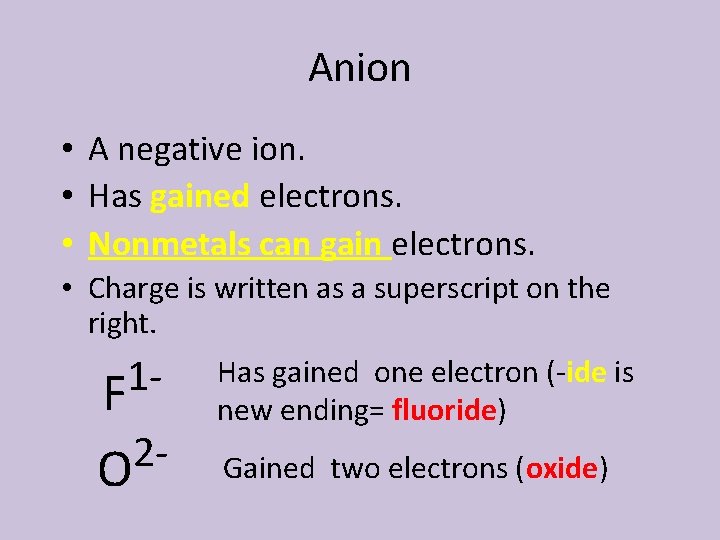
Anion • A negative ion. • Has gained electrons. • Nonmetals can gain electrons. • Charge is written as a superscript on the right. 1 F 2 O Has gained one electron (-ide is new ending= fluoride) Gained two electrons (oxide)

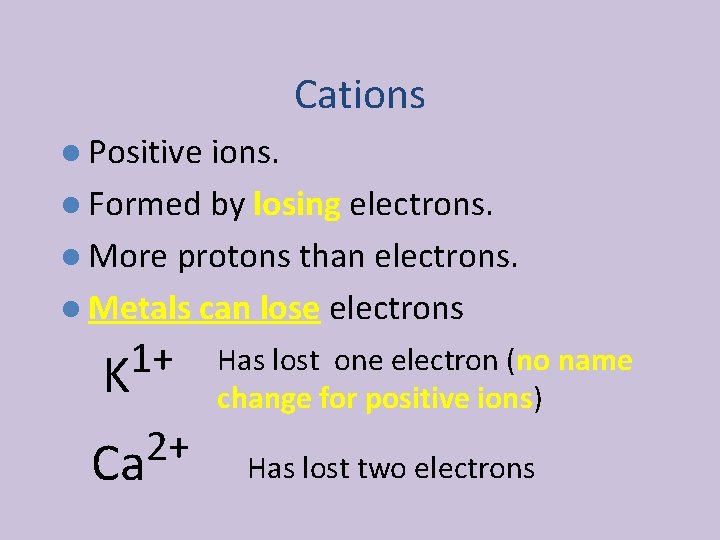
Cations l Positive ions. l Formed by losing electrons. l More protons than electrons. l Metals can lose electrons 1+ K 2+ Ca Has lost one electron (no name change for positive ions) Has lost two electrons

Ionic Compounds • Ionic compounds- from joining metal cations and nonmetal anions- to become electrically neutral • Usually solid crystals • Melt at high temperatures

Ionic Compounds • This formula represents, a formula unit • The smallest whole number ratio of atoms in an ionic compound. • Ions surround each other so you can’t say which is hooked to which.

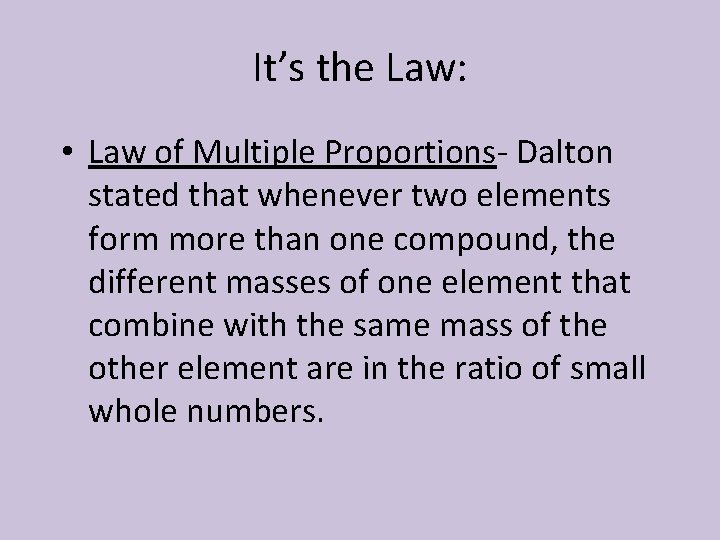
It’s the Law: • Law of Multiple Proportions- Dalton stated that whenever two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.

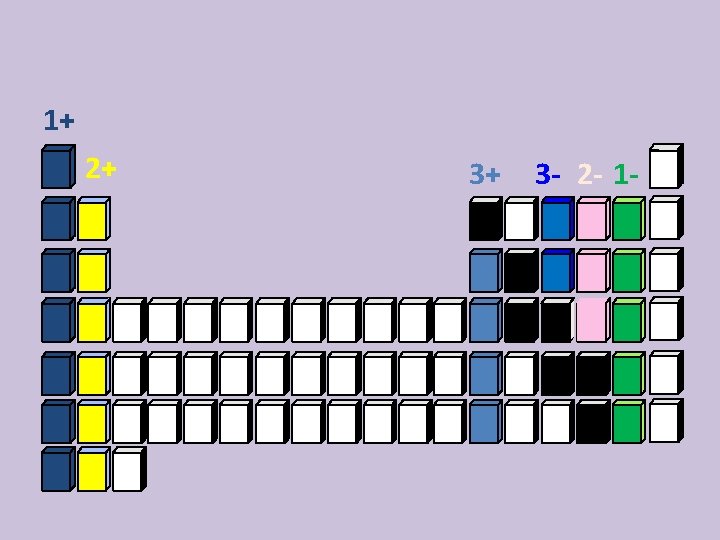
Charges on ions • For most of the Group A elements, the Periodic Table can tell what kind of ion they will form from their location; monatomic ions • Elements in the same group have similar properties. • Including the charge when they are ions.


Determining Charges or Oxidation Numbers on Cations and Anions • Group 1 A 1+ • Group 2 A 2+ LOSE to make 8 • Group 3 A 3+ electrons • Group 4 A 4+ or 4– • • Group 5 A Group 6 A Group 7 A Group 8 A 9/18/2020 3– 2– 1– 0 GAIN to make 8 electrons 13

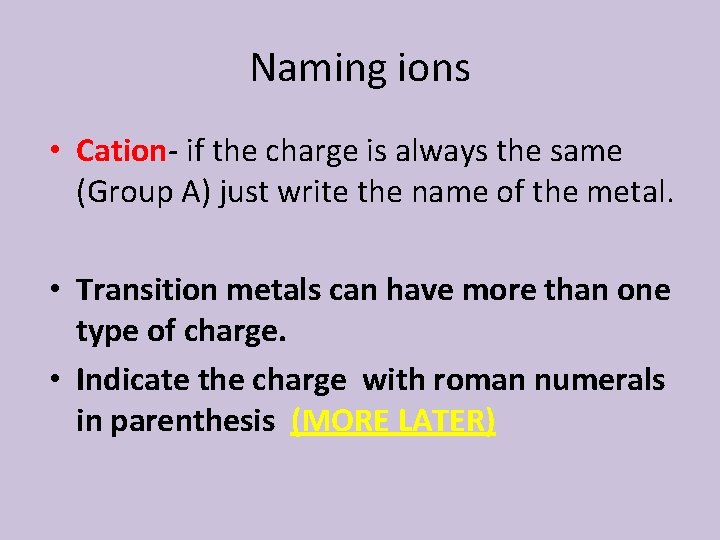
Naming ions • Cation- if the charge is always the same (Group A) just write the name of the metal. • Transition metals can have more than one type of charge. • Indicate the charge with roman numerals in parenthesis (MORE LATER)

Exceptions: • Three of the transition metals have only one ionic charge: – Do not use roman numerals for these: – Silver is always 1+ (Ag 1+) – Cadmium and Zinc are always 2+ (Cd 2+ and Zn 2+)

Name these • • Na 1+ Ca 2+ Al 3+ Cd 2+ Sr 2+ Rb 2+ Li 1+

Write Formulas for these • • • Potassium ion Magnesium ion Cesium ion Zinc ion Barium ion Aluminum ion

Naming Anions • Anions are always the same charge • Change the element ending to – ide • F 1 - Fluorine

Naming Anions • Anions are always the same charge • Change the element ending to – ide • F 1 - Fluoride

Name these • • • Cl 1 N 3 Br 1 O 2 Al 3+

Write these • • Sulfide ion iodide ion phosphide ion Cadmium ion

Naming Binary Ionic Compounds Binary ionic compounds are named by using the name of the cation first and then the anion The name of the anion will be made from the root of the element’s name plus the suffix “ide” 9/18/2020 22

Examples of Binary Ionic Compounds Zn. O = zinc oxide Na. Cl = sodium chloride Mg. Br 2 = magnesium bromide *In naming ionic compounds, the subscript number is ignored. 9/18/2020 23

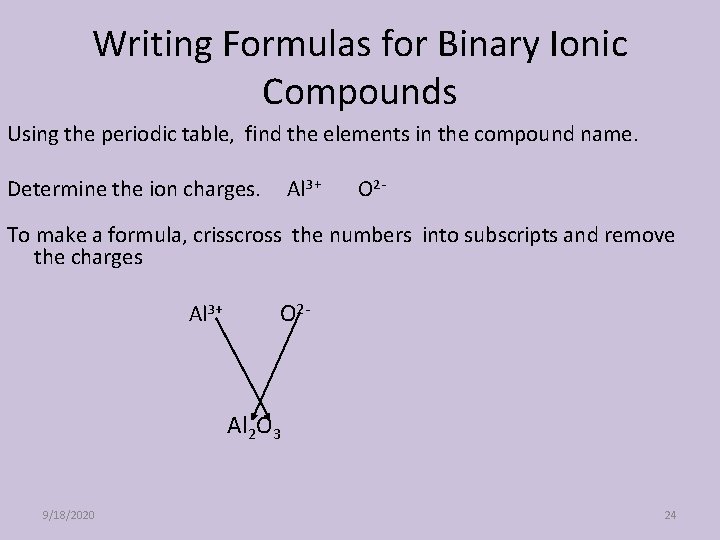
Writing Formulas for Binary Ionic Compounds Using the periodic table, find the elements in the compound name. Determine the ion charges. Al 3+ O 2 - To make a formula, crisscross the numbers into subscripts and remove the charges Al 3+ O 2 - Al 2 O 3 9/18/2020 24
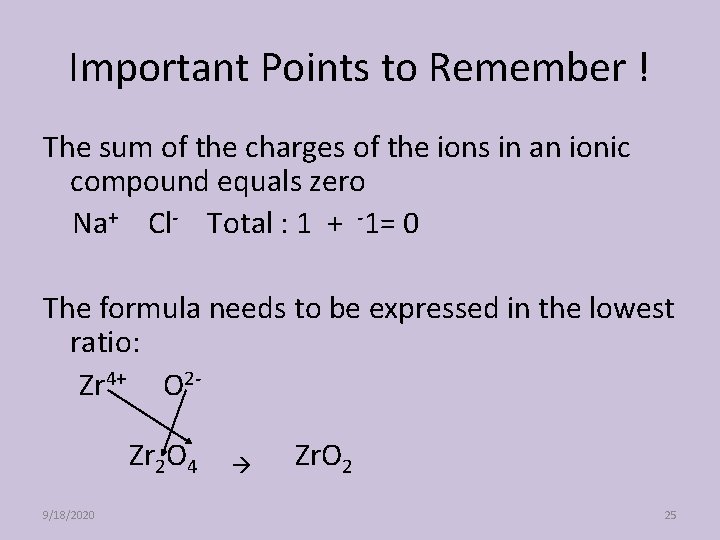
Important Points to Remember ! The sum of the charges of the ions in an ionic compound equals zero Na+ Cl- Total : 1 + -1= 0 The formula needs to be expressed in the lowest ratio: Zr 4+ O 2 Zr 2 O 4 9/18/2020 Zr. O 2 25

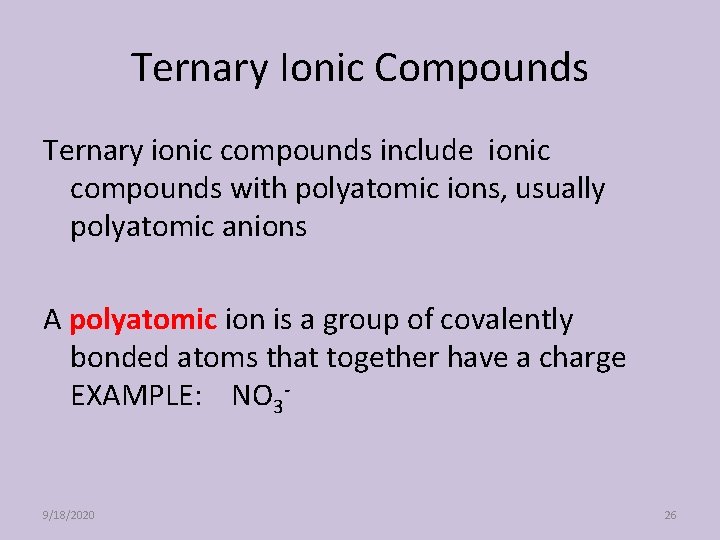
Ternary Ionic Compounds Ternary ionic compounds include ionic compounds with polyatomic ions, usually polyatomic anions A polyatomic ion is a group of covalently bonded atoms that together have a charge EXAMPLE: NO 3 - 9/18/2020 26

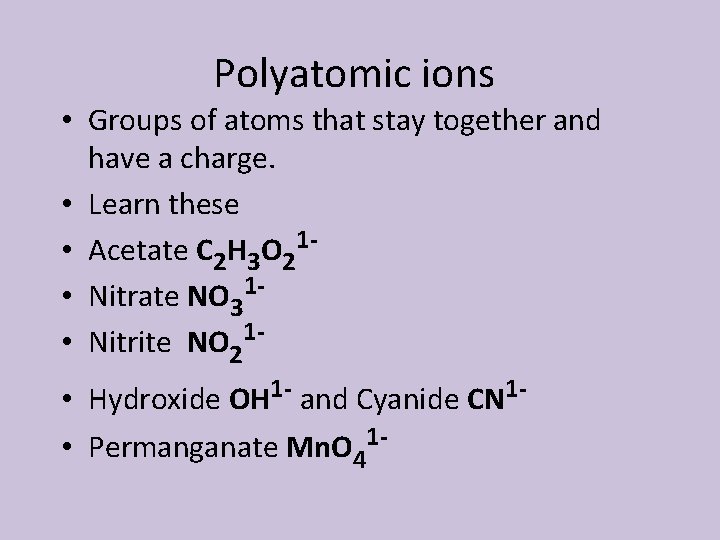
Polyatomic ions • Groups of atoms that stay together and have a charge. • Learn these 1 • Acetate C 2 H 3 O 2 • Nitrate NO 311 • Nitrite NO 2 • Hydroxide OH 1 - and Cyanide CN 1 • Permanganate Mn. O 41 -

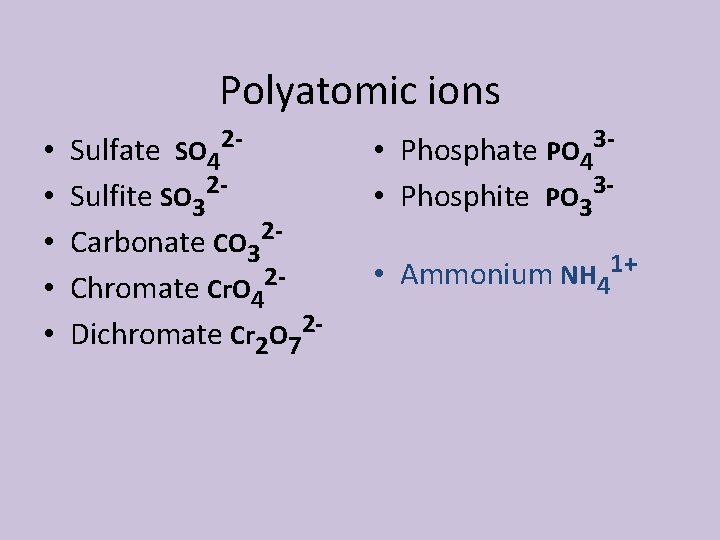
Polyatomic ions • • • Sulfate SO 42 Sulfite SO 32 Carbonate CO 32 Chromate Cr. O 42 Dichromate Cr 2 O 72 - • Phosphate PO 43 • Phosphite PO 33 • 1+ Ammonium NH 4

Ternary Ionic Compounds • • These will have polyatomic ions At least three elements name the ions Na. NO 3 Ca. SO 4 Mg. SO 3 (NH 4)2 O

Ternary Ionic Compounds • • Li. CN Al(OH)3 (NH 4)2 CO 3 Al. PO 4

Things to look for • If cations have ( ), the number in parenthesis is their charge. • If anions end in -ide they are probably off the periodic table (Monoatomic) • If anion ends in -ate or -ite it is polyatomic

Roman Numeral Binary Ionic Compounds If the cation is a representative block element, the name of the cation will be the name of the element. However, if the cation is a transition metal, you need to determine whethere is more than one charge for the element. EXAMPLE: Fe 2+ or Fe 3+ Then put the number after the name of the element in Roman numerals Fe 3+ = Iron (III) Fe. Cl 3 = Iron (III) chloride This is the Stock System of Nomenclature. 9/18/2020 32