Unit Test Naming and writing formulas What to







- Slides: 17

Unit Test: Naming and writing formulas �What to study: �Review all Bellwork questions. �Read chapter 7 section 1, 2, and 3. �Read chapter 8 section 1 and 2 �Review all key vocabulary and notes. �Practice worksheets. �Unit Test: 45 multiple choice questions. (135 pts ) �Bring a #2 pencil.

Review Chapter 7 and 8 �Naming and writing ionic Compounds �Naming and writing Transition Metals �Lewis Dot Structure �Naming Binary Molecular Molecules �Naming and writing acids

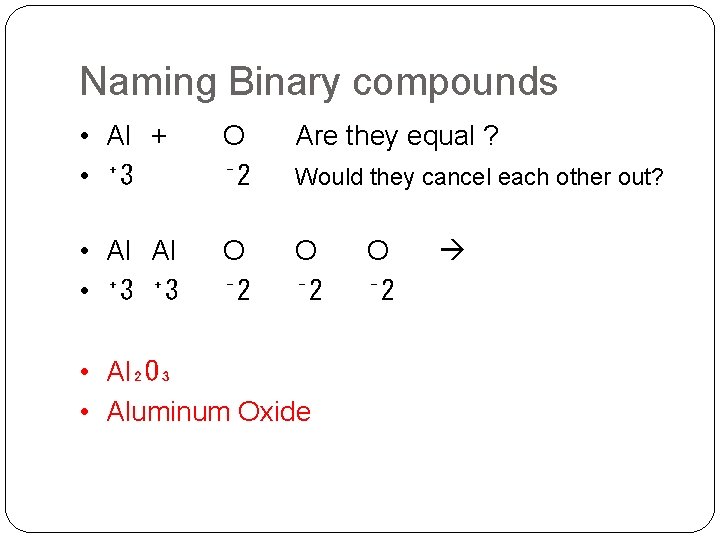
Naming Binary compounds • Al + • ⁺ 3 O ⁻ 2 Are they equal ? • Al Al • ⁺ 3 O ⁻ 2 Would they cancel each other out? • Al₂O₃ • Aluminum Oxide O ⁻ 2

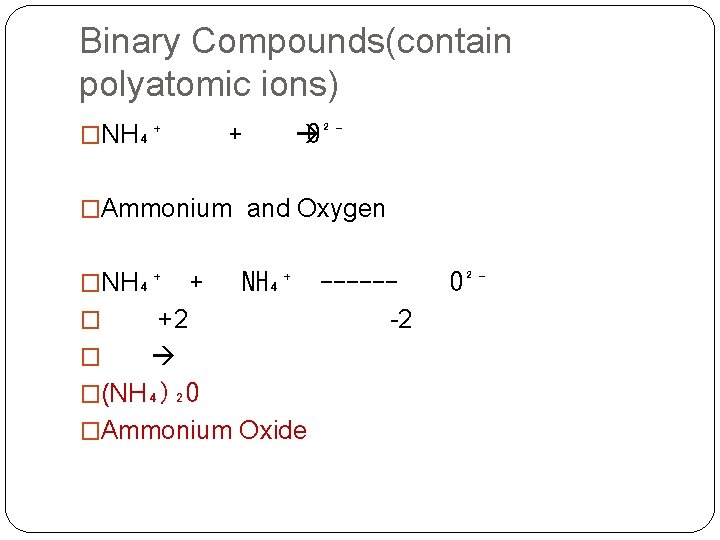
Binary Compounds(contain polyatomic ions) �NH₄⁺ + O²⁻ �Ammonium and Oxygen �NH₄⁺ + NH₄⁺ -----� +2 -2 � �(NH₄)₂O �Ammonium Oxide O²⁻

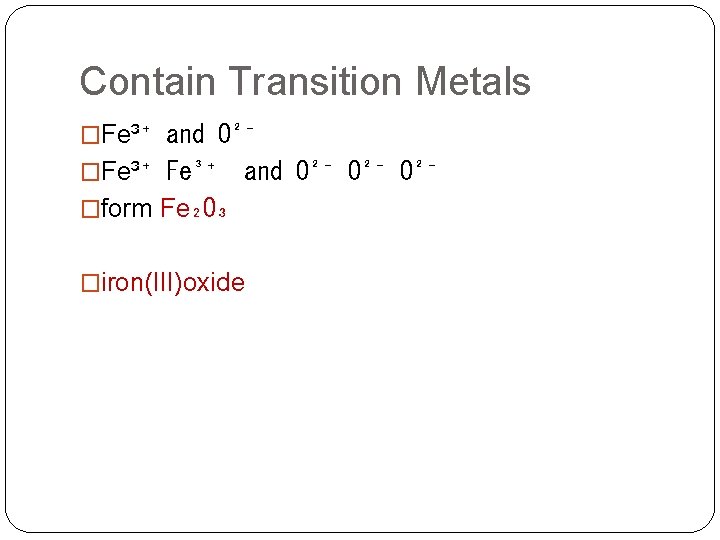
Contain Transition Metals �Fe³⁺ and O²⁻ O²⁻ �form Fe₂O₃ �iron(III)oxide

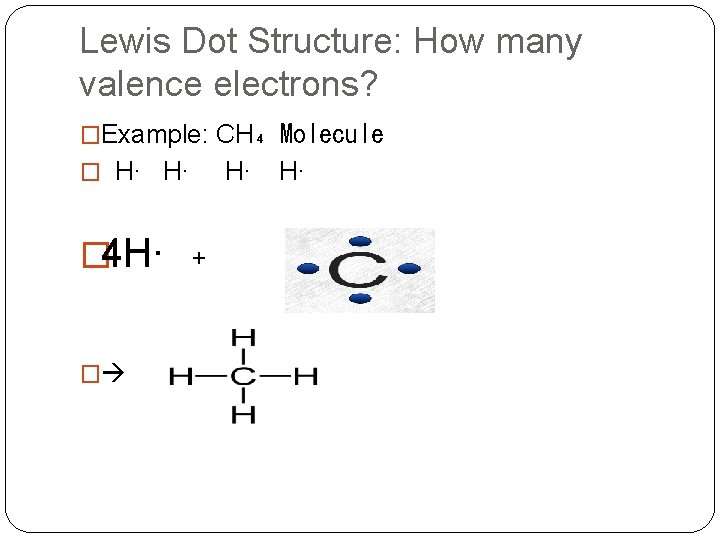
Lewis Dot Structure: How many valence electrons? �Example: CH₄ Molecule � H∙ H∙ � 4 H∙ + �

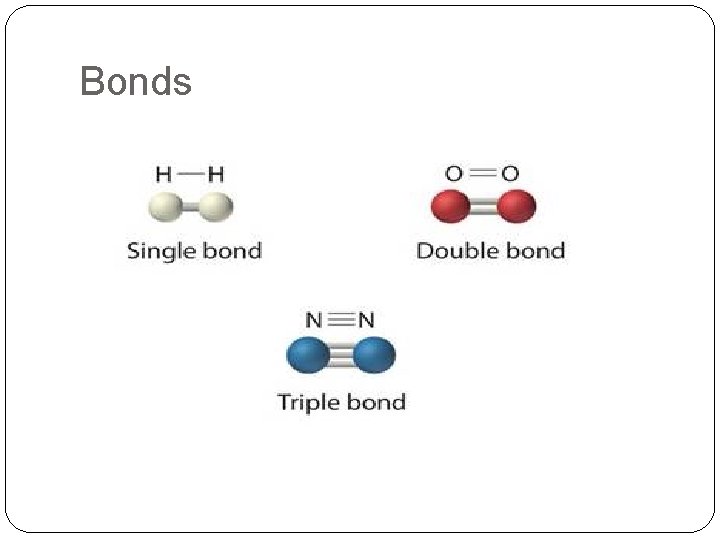
Bonds

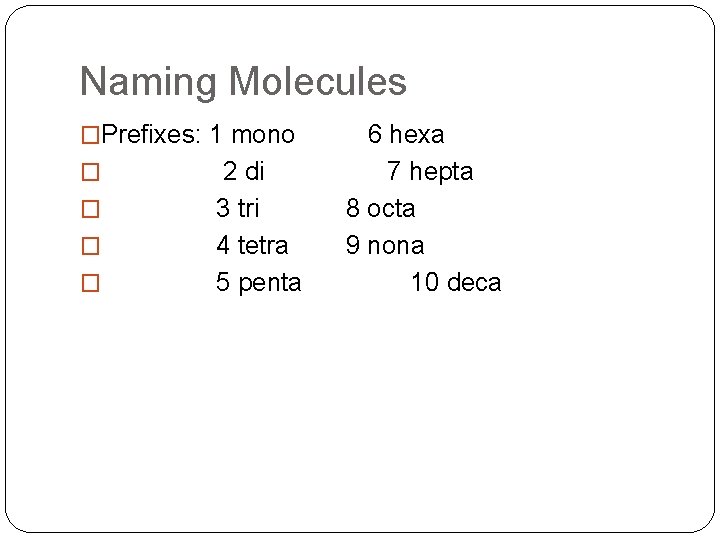
Naming Molecules �Prefixes: 1 mono 6 hexa � 2 di 7 hepta � � � 3 tri 8 octa 4 tetra 9 nona 5 penta 10 deca

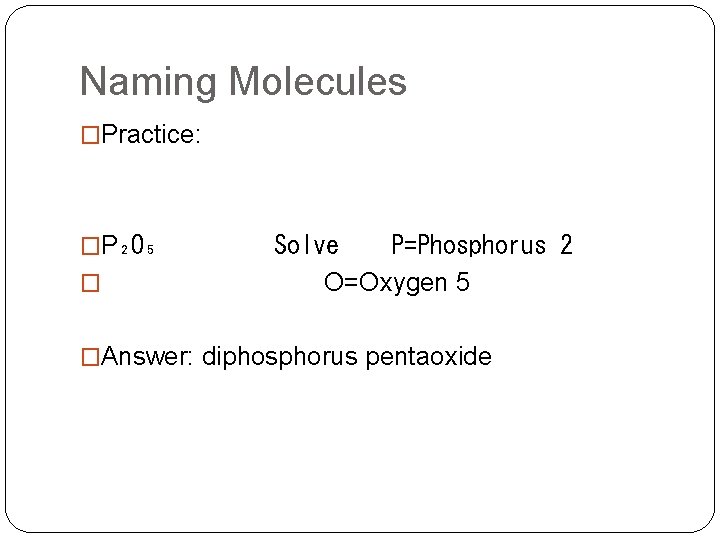
Naming Molecules �Practice: �P₂O₅ Solve P=Phosphorus 2 � O=Oxygen 5 �Answer: diphosphorus pentaoxide

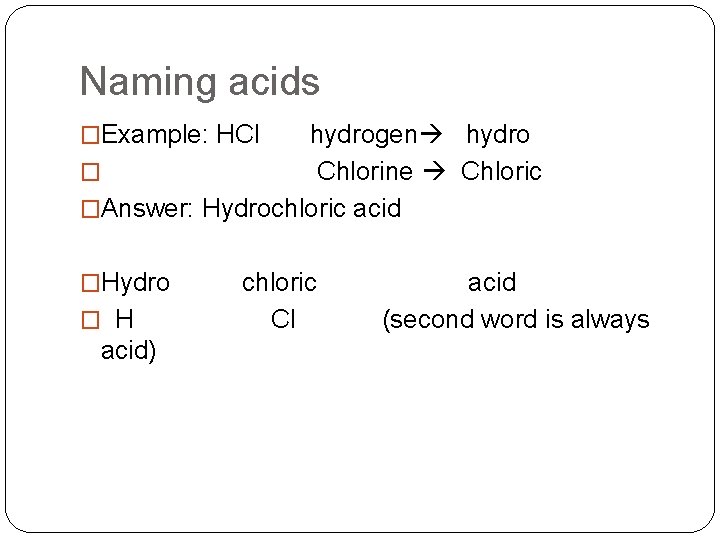
Naming acids �Example: HCl hydrogen hydro � Chlorine Chloric �Answer: Hydrochloric acid �Hydro chloric acid � H Cl (second word is always acid)

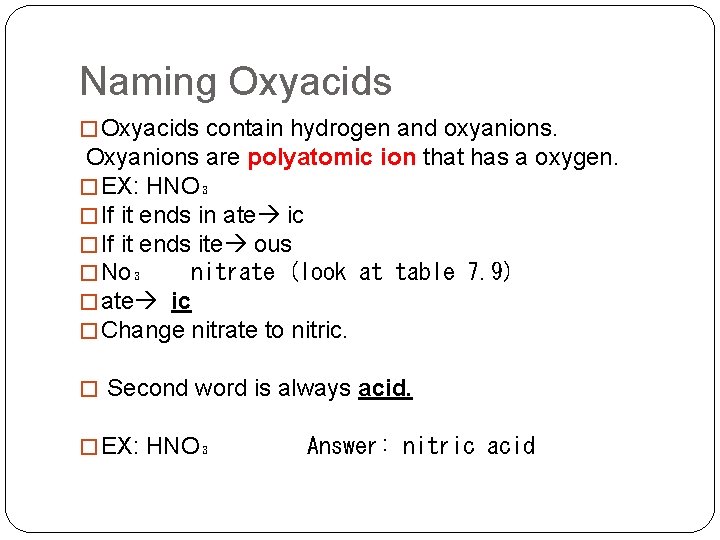
Naming Oxyacids � Oxyacids contain hydrogen and oxyanions. Oxyanions are polyatomic ion that has a oxygen. � EX: HNO₃ � If it ends in ate ic � If it ends ite ous � No₃ nitrate (look at table 7. 9) � ate ic � Change nitrate to nitric. � Second word is always acid. � EX: HNO₃ Answer: nitric acid

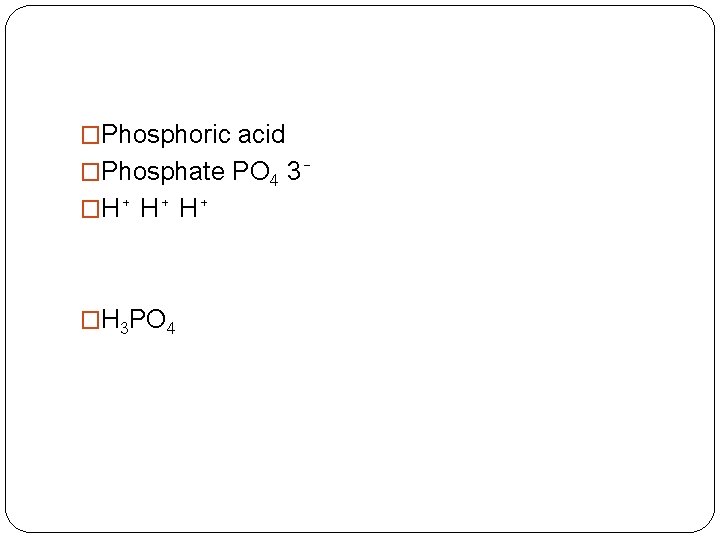
�Phosphoric acid �Phosphate PO 4 3⁻ �H⁺ H⁺ H⁺ �H 3 PO 4

Key vocabulary for unit test �Chemical bond �Cation �Anion �Ionic bond �Ionic compound �Binary Compounds �Transition Metals �Lewis Dot Structure �Molecules compounds

�Monoatomic ion �Formula unit �Oxidation number �Polyatomic ion �Oxyanion �Covalent bond �Oxyacids �Binary acids �Octet Rule �Valence electrons

�Single covalent bond �Double covalent bond �Triple covalent bond

Practice: � Name the following chemical compounds: � 1) Na. Br � 2) Ca(C 2 H 3 O 2)2 � 3) P 2 O 5 � 4) Ti(SO 4)2 � 5) Fe. PO 4 � 6) K 3 N � 7) SO 2 � 8) Cu. OH � 9) Zn(NO 2)2 � 10) V 2 S 3

Practice: Write The Formula �Write the formulas for the following chemical compounds: � 11) silicon dioxide � 12) nickel (III) sulfide � 13) manganese (II) phosphate � 14) silver acetate � 15) diboron tetrabromide � 16) magnesium sulfate heptahydrate � 17) potassium carbonate � 18) ammonium oxide � 19) tin (IV) selenide