The Office of Research Integrity and Assurance ORIA















































- Slides: 60

The Office of Research Integrity and Assurance (ORIA) https: //researchintegrity. asu. edu/home 1

Ask Questions at Any Time ked. asu. edu 2

About the Presenter Erik Williams § Certified IRB Professional § IRB Coordinator § ASU § Previous: IRB Board Member § U of Wisconsin: Eau Claire § Erik. B. Williams@ASU. EDU ked. asu. edu 3

Overview + Definitions https: //researchintegrity. asu. edu/home 4

Overview Biosafety Human Subjects Research Cultural Review ORIA Animal Care and Use Scientific Diving Security and Export Controls “The Institutional Review Board” (IRB) The IRB reviews all proposed Research involving Human Subjects to ensure that subjects are treated ethically and that their rights and welfare adequately protected. Responsible Conduct in Research https: //researchintegrity. asu. edu/home 5

Overview What is an IRB? § The Board | The Office (ORIA) (for the sake of ease, both are “the IRB”) § Reviews are conducted by individual members or by full board meeting + vote (depending on risk) § Boards: diverse membership requirements § Men + Women | Various disciplines | Scientists + Non-scientists Prisoner Representative | At Least One Community Member § Two boards @ ASU: Bioscience | Social Behavioral § Overseen by ORHP (DHHS), FDA regulations (rarely others) ked. asu. edu 6

Definitions What is Human Subjects Research? Research & Human Subject “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge” “a living individual about whom an investigator conducting research obtains data through intervention or interaction with the individual or identifiable private information” ked. asu. edu 7

History of Human Rights Violations “Errors of Enthusiasm” https: //researchintegrity. asu. edu/home 8

History of Human Rights Violations (a few of the “big ones”): Nazi War Crimes (1940 s) US Human Radiation experiments (1944 -1974) The Jewish Chronic Disease Hospital Study (1963) The Willowbrook Study (1956 -71) Stanford Prison Experiment (1971) ked. asu. edu 9

The Nuremberg Code ked. asu. edu 10

History The Nuremberg Code In 1947, in response to Nazi ‘science’, the Nuremberg Code for research on human subjects is adopted. The Allies convicted 23 Nazi scientists of war crimes at the Nuremberg Trials. 10 Key Elements: § Voluntary consent § Anticipate scientific benefits § Benefits must outweigh risks § Perform animal experiments first § Avoid suffering § No intentional death or disability § Do no harm § Subjects can withdraw at any time § Investigators must be qualified § Research will stop if harm occurs ked. asu. edu 11

Brief History of Human Rights Violations after Nuremberg Henrietta Lacks (1951) § Cells were harvested without permission. Cells were cultured and created first human immortal cell line. The Jewish Chronic Disease Hospital Study (1963) § Patients were injected with live cancer cells. Consent did not include any discussion on the injection of cancer cells. The Willowbrook Study (1956 -71) § “Mentally defective” children were injected with Hepatitis. Parents were coerced into agreeing in order to have their children admitted. Monster Study on Stuttering (1939) | Guatemala STD Experiments (1946) | MIT Science Club Study (1946 -53) | Sloan-Kettering Cancer Study (1952) | Wichita Jury Study (1953) | Military LSD experiments (1953) | Thalidomide Tragedy (1957) | Harvard Psilocybin Experiments (1960 -63) | Milgram Obedience Study (1962) | Jewish Chronic Disease Hospital Cancer Study (1963) | Beecher Article (1966) | Tearoom Trade Study published (1970) | Stanford Prison Experiment (1971). … http: //sites. jcu. edu/research/pages/irb/help/irb-timeline/ ked. asu. edu 12

Tuskegee Syphilis Study ked. asu. edu 13

Tuskegee Syphilis Study 1932 to 1972 § Government-run project (US Public Health Service) w/Tuskegee U. § Hundreds of impoverished black sharecroppers told they were being treated for “bad blood”. No diagnoses were disclosed and no treatment was given. § 1947: penicillin had become standard treatment for syphilis. Scientist continue to withhold and prevent access to treatment. § 1972: a leak resulted in study termination. § Led to Belmont Report (1976) and development of IRBs § President Clinton issued 1 st formal apology in 1997. ked. asu. edu 14

Tuskegee Real and Long-term Impact “We find that the historical disclosure of the study in 1972 is correlated with increases in medical mistrust and mortality and decreases in both outpatient and inpatient physician interactions for older black men. Our estimates imply life expectancy at age 45 for black men fell by up to 1. 4 years in response to the disclosure, accounting for approximately 35% of the 1980 life expectancy gap between black and white men. ” Tuskegee and the Health of Black Men, June 2016 ked. asu. edu 15

Two Overlapping Regulations: Belmont Report (1979) & The Common Rule (1981) ked. asu. edu 16

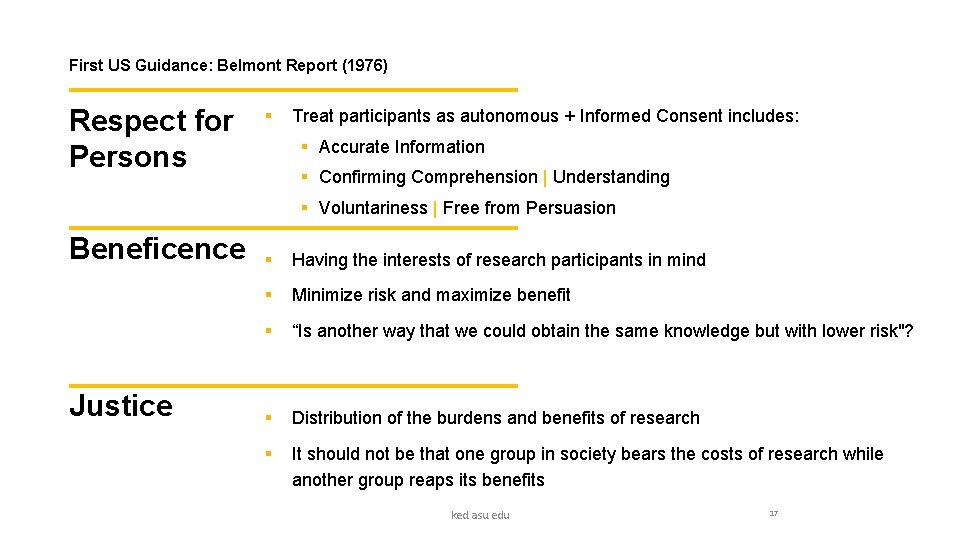
First US Guidance: Belmont Report (1976) Respect for Persons § Treat participants as autonomous + Informed Consent includes: § Accurate Information § Confirming Comprehension | Understanding § Voluntariness | Free from Persuasion Beneficence Justice § Having the interests of research participants in mind § Minimize risk and maximize benefit § “Is another way that we could obtain the same knowledge but with lower risk"? § Distribution of the burdens and benefits of research § It should not be that one group in society bears the costs of research while another group reaps its benefits ked. asu. edu 17

First US Regulation: Common Rule (1981) The Common Rule (1981) Describe processes and regulations. Ethics and morality takes ‘back-burner’ and is addressed in the Belmont Report. § 45 CFR 46 Federal Policy for the Protection of Human Subjects § 21 CFR 50 FDA-regulated clinical investigations § 21 CFR 56 Responsibility of IRBs that review FDA-regulated clinical investigations Updated in 1991 and again in 2017. ked. asu. edu 18

Research Misconduct After Regulations: New Challenges “Errors of Enthusiasm” ked. asu. edu 19

Misconduct After Regulations: New Challenges – a few examples 1989 – ASU Havasupai Research Study § ASU Researchers collected blood samples for a Diabetes study in 1989. § Samples were later used for studies on schizophrenia, migration, and inbreeding, all of which are taboo topics for the Havasupai. The Tribe sued ASU in 2004 after finding out. ked. asu. edu 20

Misconduct After Regulations : New Challenges – a few examples 2014 – Facebook Emotional Cognition Study § Facebook manipulated news feeds in order to detect changes in participant emotions. 2016 – Ok. Cupid Data Breach § Data scraped from 70, 000 Ok. Cupid dating profiles. Easy to re-identify. § Including their usernames, age, gender, location, what kind of relationship (or sex) they’re interested in, personality traits, and answers to thousands of profiling questions used by the site. For more details + history, see http: //sites. jcu. edu/research/pages/irb/help/irb-timeline/ ked. asu. edu 21

IRB Review @ ASU ked. asu. edu 22

What is the IRB’s Purview and Mandate? § The ASU IRB approves ASU affiliated students, faculty, and staff to conduct specific procedures using specific materials for research purposes. § We ensure that these procedures and materials adhere to ethical standards and local, state, and federal regulations. ked. asu. edu 23

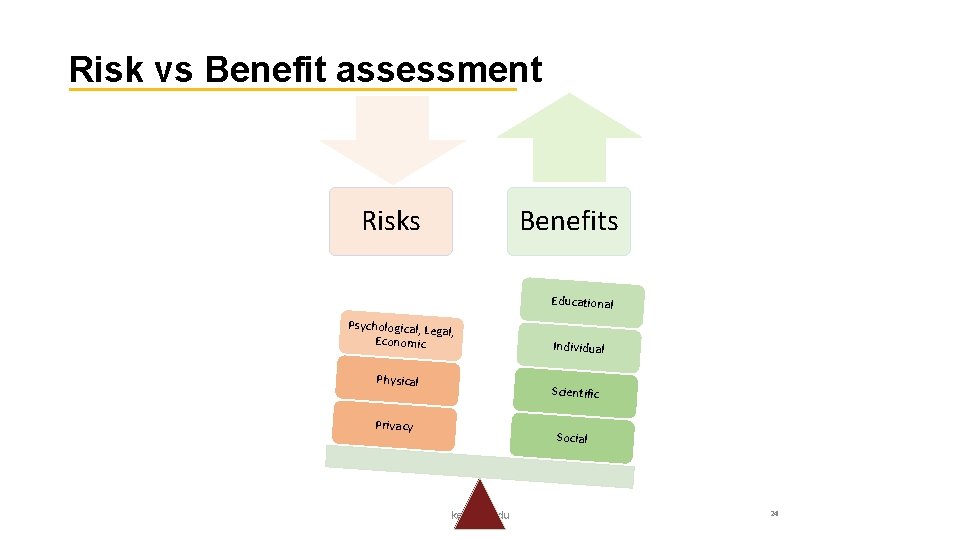
Risk vs Benefit assessment Risks Benefits Educational Psychological, Lega l, Economic Physical Individual Scientific Privacy Social ked. asu. edu 24

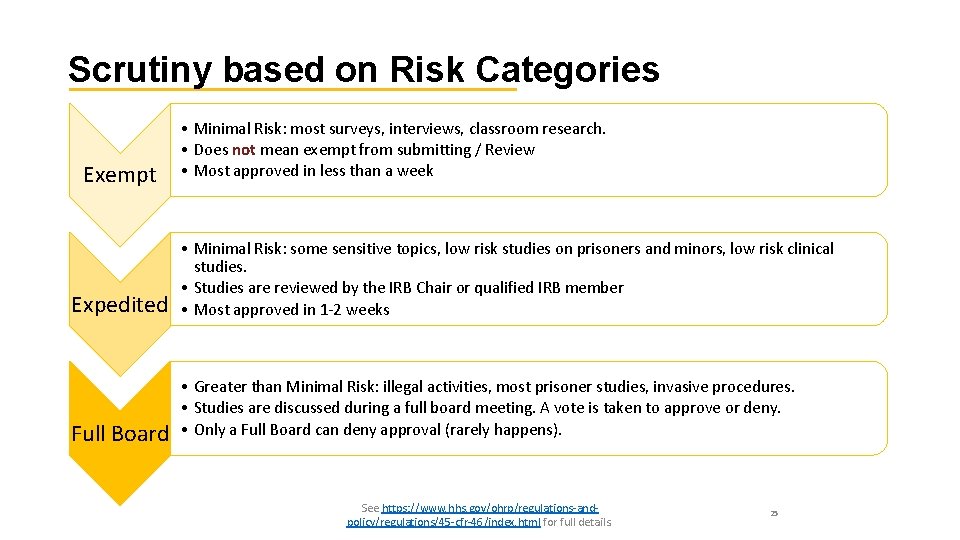
Scrutiny based on Risk Categories Exempt • Minimal Risk: most surveys, interviews, classroom research. • Does not mean exempt from submitting / Review • Most approved in less than a week Expedited • Minimal Risk: some sensitive topics, low risk studies on prisoners and minors, low risk clinical studies. • Studies are reviewed by the IRB Chair or qualified IRB member • Most approved in 1 -2 weeks Full Board • Greater than Minimal Risk: illegal activities, most prisoner studies, invasive procedures. • Studies are discussed during a full board meeting. A vote is taken to approve or deny. • Only a Full Board can deny approval (rarely happens). See https: //www. hhs. gov/ohrp/regulations-andpolicy/regulations/45 -cfr-46/index. html for full details. 25

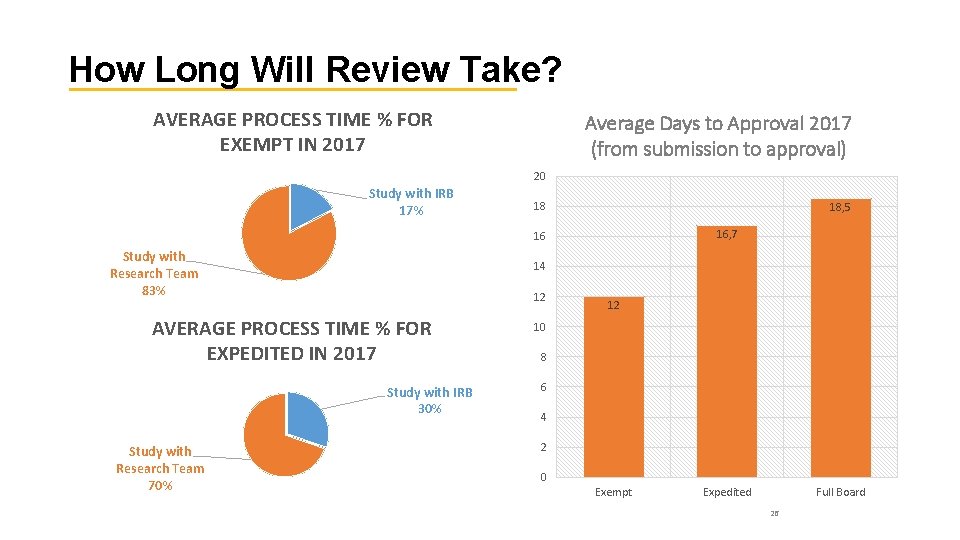
How Long Will Review Take? AVERAGE PROCESS TIME % FOR EXEMPT IN 2017 Average Days to Approval 2017 (from submission to approval) 20 Study with IRB 17% 18 18, 5 16, 7 16 Study with Research Team 83% 14 12 AVERAGE PROCESS TIME % FOR EXPEDITED IN 2017 Study with IRB 30% Study with Research Team 70% 12 10 8 6 4 2 0 Exempt Expedited Full Board 26

Review @ ASU When Do I Need To Submit? At ASU, any projects which meet the definition of Human Subjects Research must be reviewed + approved prior to any recruitment or data collection. ked. asu. edu 27

Review @ ASU Flashback: Definition of Human Subjects Research & Human Subject “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge” “a living individual about whom an investigator conducting research obtains data through intervention or interaction with the individual or identifiable private information” ked. asu. edu 28

Review @ ASU ked. asu. edu 29

Definitions Study Team Member A study team member is anyone Engaged in Research defined as: Actively consenting participants | Involved with collecting data Having access to raw data | Analyzing raw data § Must be added as a “Study Team Member” and complete CITI training. Principal Investigator (PI) Faculty or full-time staff member who assumes the following responsibilities: § Submitting materials + Submitting Modifications + Reporting Problems § Conduct of the research § Compliance with IRB decisions ked. asu. edu 30

CITI Training • All study team members must complete CITI training: IRB - Biomedical Research (Group 1) OR; IRB - Social & Behavioral Research (Group 2) • About 1 -3 hours. • Several modules which consist of a few paragraphs and a short quiz. • Progress is saved after each module. • www. citiprogram. org 31

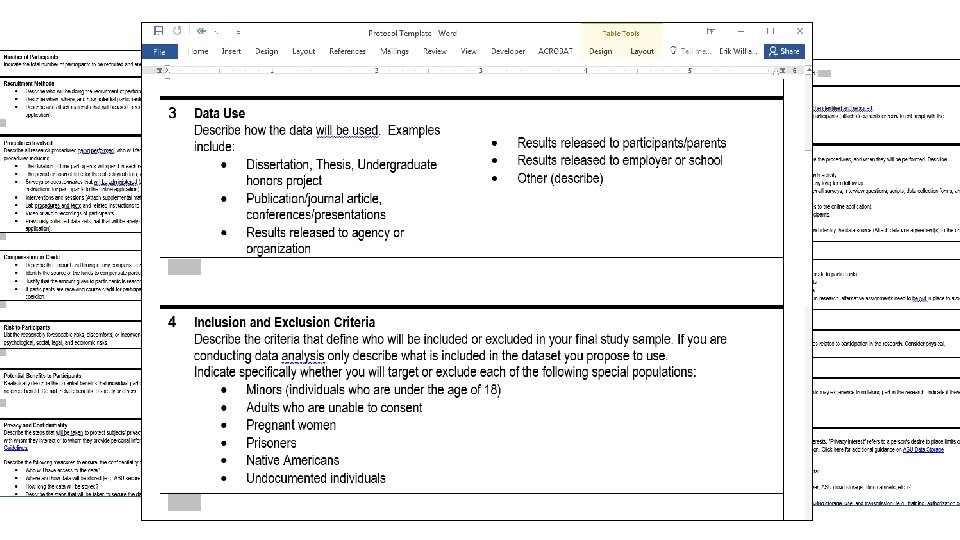
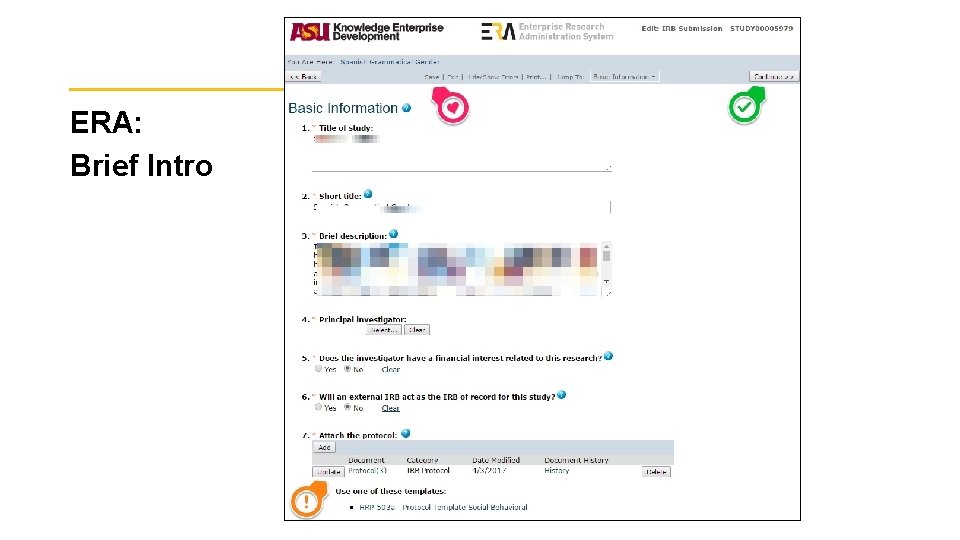
Writing the Protocol Document • Contextualize and brings together all aspects of the research for the IRB and is a road map for everything else. Outline the work conducted for research purposes. Describe ALL aspects of your project consistently in it. • Avoid jargon. Write clearly and plainly. Use charts, grids, diagrams, etc when they help explain. • When describing procedures/processes: • Too Vague: we won’t understand what you will be doing or how. • Too Specific: you may need unnecessary modifications or may become non-compliant. • Find your ‘Goldilocks’: Just Right! 32

33

Writing the Consent Form • The most important part of informed consent is to clearly explain what you will do in a manner that is easy for participants to understand. • There a number of required elements of consent – consult our templates for a summary of these. • You do not need to use our templates. The most effective consent forms are often ones developed by research teams. • Informed consent is NOT a contract or binding agreement. 34

Do I Need Signed Consent Studies that meet the following criteria don’t typically require signed consent: • Participants are all 18 or older. • No identifying information is collected in any way. • Are a one-time survey or individual interview. Most other studies will require signed consent. These include: focus groups, studies that involve minors (parental consent), requests to use grades/other nonresearch data, biomedical procedures, etc. 35

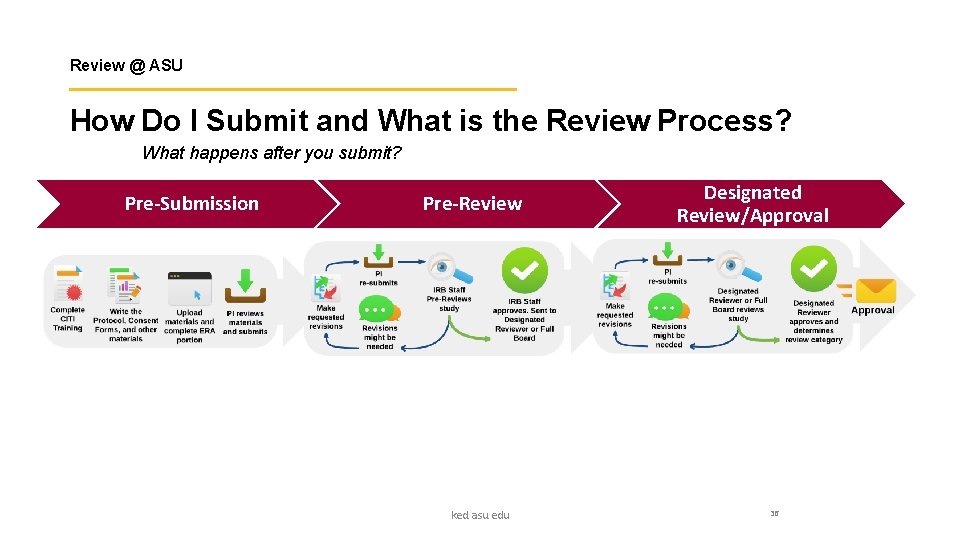
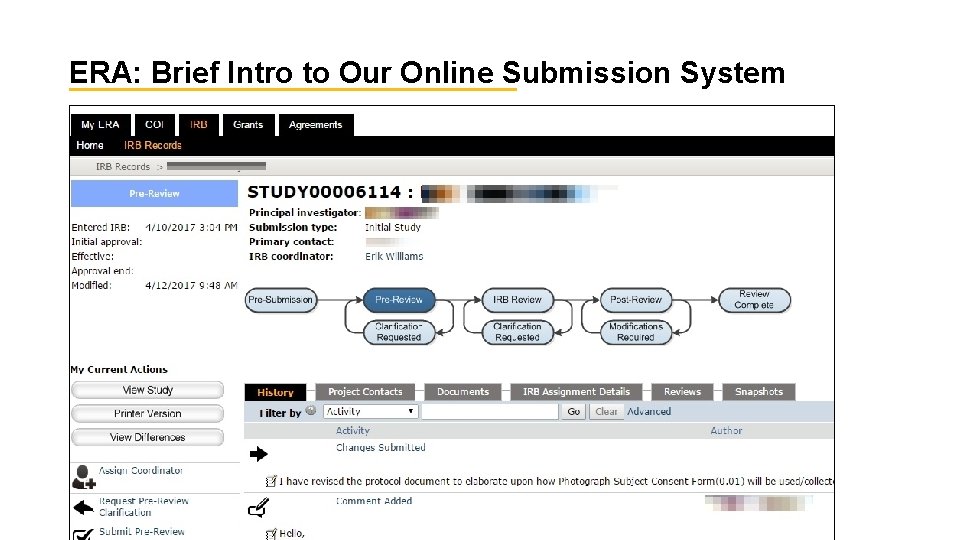
Review @ ASU How Do I Submit and What is the Review Process? What happens after you submit? Pre-Submission Pre-Review ked. asu. edu Designated Review/Approval 36

ERA: Brief Intro to Our Online Submission System 37

ERA: Brief Intro 38

Modifications § Most studies will require any changes to be approved before implementing. § Changes can include: addition of study team members, removal of procedures, adding on phases to the project, tweaking wording. § A modification cannot be implemented until it is approved. Your approved study should always reflect the current research activities. § Multiphase studies can be “built up” via multiple modifications. § Mods exist as a “sub study” before being approved. Review process is similar to a new study. § Sometimes it is simpler or ‘cleaner’ to submit a new study. ked. asu. edu 39

Continuing Reviews and Study Closure § Full Board studies are approved for 1 year + require annual Continuing Reviews to be submitted + approved. § Some Expedited studies will require an annual continuing review as well (new regulations). § Annual Continuing Reviews are very simple, but give 7 days to review. Your study may lapse if you submit the day of your expiration date. § A study is complete and should be closed when data collection + data analysis are complete. ked. asu. edu 40

Research on your own students/Extra Credit • A status relationship – can also apply to students, employees, or family. • Potential for perception of coercion. • Address status relationship during consent process. • Have someone else collect data and blind you to who participated. • Recruit and collect data after grades have been given out. • Why do you need to study your own students? • High risk or high burden projects are of more concern. • Extra Credit: • Have equitable (in time and effort) non-research alternative. • Participants should be able to withdraw and still receive credit. • Credit small in proportion to a student’s overall grade. • Documentation of credit does not jeopardize anonymity. See https: //researchintegrity. asu. edu/human-subjects/special-considerations

Collection secondary data: Aggregate vs Individual Level § Truly aggregate data typically does not require any consent to use in research. IRB approval may be required and you should e-mail first. § Data that is ‘individual level data’ – even if identifying information is stripped – will likely require IRB approval. § Who is aggregating or de-identifying the data? ked. asu. edu 42

Existing vs Prospective data: when is consent needed? § While you may have permissions to access data and information in order to teach, you do not necessarily have permission to use that data for research purposes. § Which role or “hat” are you wearing when accessing the data? Researcher vs instructor. § Most data collected form coursework is also protected by FERPA. § Existing data: the IRB typically requests that consent be obtained for the use of individual level data unless a clear argument can be made that the research could not practicably be carried out if consent is required. ked. asu. edu 43

Tips for an Easy Review § Keep things clear. A submission shouldn’t require guesswork to piece together. § Keep it as simple as possible. Don’t discuss epistemology or pedagogy in your protocol or consent form. Describe what you will be doing in simple and clear ways. For example, a simple explanation of “At T 3, we will administer the survey titled T 3 Survey. doc via a paper survey” is sufficient. § Submit a complete protocol. Don’t ask questions in the protocol/consent – submit with your best option and, if not appropriate, revisions will be requested. § Submit all your study instruments and materials. § Review everything for consistency. ked. asu. edu 44

Prepared Special Topics: • • • Non-ASU Collaborators/Two IRBs International Research How to formulate an IRB proposal for the purpose of gathering a corpus Confidentiality vs Anonymity Translating Resources Use of public internet postings Recruitment of research participants through students Research on your own students/Extra Credit Populations that distrust signing materials When do I need a Letter of Permission ANY other questions you may have ked. asu. edu 45

Review @ ASU Help + Resources Templates, forms, and requirements can be found on the IRB homepage: https: //researchintegrity. asu. edu/humans For additional help using ERA, please consult our webpage or contact us: https: //researchintegrity. asu. edu/human-subjects/protocol-submission ked. asu. edu 46

Misc PDFs + Resources (double click to open) ked. asu. edu 47

Misc Internet Resources The CORE Network: http: //thecore. ucsd. edu/. Mobile Imaging, pervasive Sensing, Social media and location Tracking or MISST The CORE brings researchers, ethics board members, technologists and other stakeholders together to share their expertise, resources, and experiences to shape best practices. Quorum Knowledge Center: http: //www. quorumreview. com/knowledge-center/ Focused on IRBs, but has some researcher resources. More than Meets The IRB podcast: https: //soundcloud. com/publicresponsibility Focused on discussion about research. Tends to focus on IRBs, but could be valuable to researchers. ked. asu. edu 48

Questions? ked. asu. edu 49

Thank You! Contact Information ORIA staff are always happy to answer questions and assist you. • Susan Metosky, Compliance Officer Susan. Metosky@asu. edu (480) 727 -0871 • Tiffany Dunning Tiffany. Dunning@asu. edu (480) 639 -7396 • Erik Williams Erik. b. Williams@asu. edu (480) 727 -5906 • Kaleigh Michalko Kaleigh. Michalko@asu. edu (480) 727 -5602 • General Questions Research. integrity@asu. edu ked. asu. edu 50

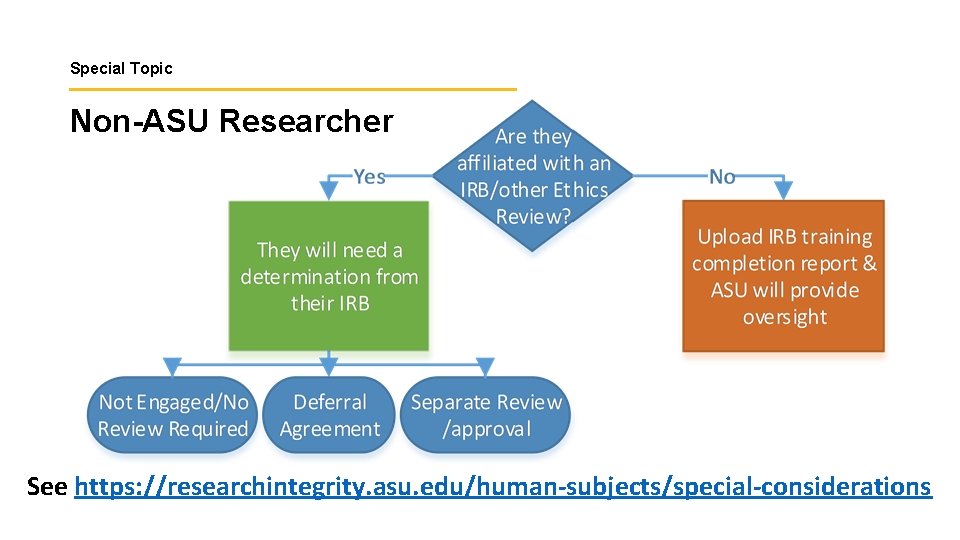
Special Topic Non-ASU Researcher See https: //researchintegrity. asu. edu/human-subjects/special-considerations

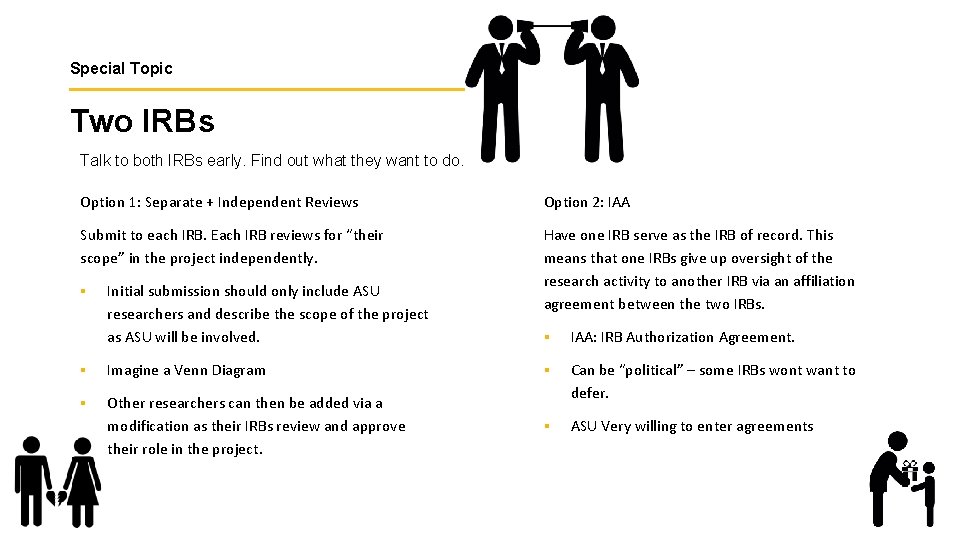
Special Topic Two IRBs Talk to both IRBs early. Find out what they want to do. Option 1: Separate + Independent Reviews Option 2: IAA Submit to each IRB. Each IRB reviews for “their scope” in the project independently. Have one IRB serve as the IRB of record. This means that one IRBs give up oversight of the research activity to another IRB via an affiliation agreement between the two IRBs. § Initial submission should only include ASU researchers and describe the scope of the project as ASU will be involved. § IAA: IRB Authorization Agreement. § Imagine a Venn Diagram § § Other researchers can then be added via a modification as their IRBs review and approve their role in the project. Can be “political” – some IRBs wont want to defer. § ASU Very willing to enter agreements

Recruitment of research participants through students • Potential risks of persuasive recruitment. • At other universities/schools – ensure you have appropriate permissions and are following local policy. • Some universities require all research *conducted* at the location to undergo IRB review. • Some universities don’t allow any recruitment of students. Others have no restrictions. See https: //researchintegrity. asu. edu/human-subjects/special-considerations

Confidentiality vs Anonymity Confidential means that only the research team can identify the responses of individual subjects; however, the researchers must make every effort to prevent anyone outside of the project from connecting individual subjects with their responses. Anonymous means that either the project does not collect identifying information of individual subjects, or the project cannot link individual responses with participants’ identities. A study should not collect identifying information of research participants unless it is essential to the study protocol. Ways to Protect Confidentiality • Limit identifying information collected to the bare minimum needed to answer your research question. • Separate identifying info from other data whenever possible. • Collect names for prizes/compensation on a separate survey, sheet or form. • Use study codes on documents and keep a list of codes connected to names separate from study data. • Link data via Anonymous Reproducible ID instead of names

Special Topic International Research § What expertise do you have to conduct research in the location or with the subject population? § What languages will be used/can be used. Are there any literacy issues? § Are you collaborating with a local institution? § Are risks and benefits different? § Other considerations: § Local age of majority § Cultural sensitivities § Autonomy regarding consent. Who is able to give consent (age, gender, etc)? § Culturally appropriate access to the community Note that it is the investigators responsibility to know and adhere to local laws/regulation. See http: //humansubjects. stanford. edu/research/documents/OHRP-intlcomp. pdf ked. asu. edu 55

Special Topic Populations that distrust signing materials § Conduct of research that is respectful of cultures. Think Havasupai. § How do you convince the IRB you ‘understand the culture’? Briefly explain the situation and why alterative procedures are needed. § Signed consent should not be waived because it is “kind of a lot of work”, but should be waived in situations where signed consent is not culturally appropriate. § Local review and cultural review processes if available. § Informed consent is a process not a document. § Study risk. ked. asu. edu 56

How to formulate an IRB proposal for the purpose of gathering a corpus • Clear consent process to ensure participants know how their data will and will not be used. • Explain: • How will data be stored? How long? Where? • How will data be used? How will it not be used? • Will data contain identifying information? Demographic information? • Difficult questions/discussion: What can benign data reveal about us? What is deidentified? What does the future hold?

Use of public internet postings • “Reasonable expectation of privacy” – what is public / what is not public. • Interaction with participants and deception. • Points to include: • Will identifiers be collected/recorded with data? • Will the source be identified in publications? • Is the data public or are there any expectations of privacy? • Visit the CORE Network: http: //thecore. ucsd. edu/. Tons of great resources,

Special Topic Translating Resources Initial Submission should include: § English versions of all materials. § An explanation of what languages it will need to be translated into in the protocol. After English Versions are Ready we will prompt you to upload: § Copies of translated materials (do not upload the “back translated” materials). § A copy of the Translation Certification Form signed by the translator and back translator. § Translators can typically be anyone fluent in language (no certification requirements/etc) See https: //researchintegrity. asu. edu/human-subjects/protocol-submission

Special Topic Q: When do I need a Letter of Permission A: It Depends • Any time research occurs in a clinic and collects data from/about patients. • Research collecting data from/about adult employees only may not require a letter, though you will want to consult with the site to ensure it is OK. Particularly if you are using resources from the site for recruitment. • Any time research occurs in a non-ASU school and collects data from/about the students. • Research collecting data from/about the teachers may not require a letter, though you will want to consult with the site to ensure it is OK. • Generally, if it is a setting where an average individual “off the street” could not walk into and begin interacting with the individuals, a letter of permission will be requested.
 Alex standards science
Alex standards science Ies pardo tavera
Ies pardo tavera Doctor eugenio oria
Doctor eugenio oria Cardenal es una palabra aguda grave o esdrujula
Cardenal es una palabra aguda grave o esdrujula Ceip cardenal herrera oria
Ceip cardenal herrera oria Methodological integrity in qualitative research
Methodological integrity in qualitative research Uq research integrity module
Uq research integrity module Epigeum research integrity training
Epigeum research integrity training Difference between office location and office layout
Difference between office location and office layout Quality assurance theory
Quality assurance theory Quality revolution
Quality revolution Software testing and quality assurance theory and practice
Software testing and quality assurance theory and practice Software testing and quality assurance theory and practice
Software testing and quality assurance theory and practice Software testing and quality assurance theory and practice
Software testing and quality assurance theory and practice Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V cc cc
V cc cc Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Số nguyên tố là số gì
Số nguyên tố là số gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Intellectual intergrity
Intellectual intergrity Mistrust examples
Mistrust examples Database security and auditing
Database security and auditing School integrity, commitment and loyalty are
School integrity, commitment and loyalty are Honesty integrity and reliability
Honesty integrity and reliability