Research Integrity Governance Outline Scope of Research Integrity

















- Slides: 19

Research Integrity & Governance

Outline • Scope of Research Integrity and Governance • Research governance in our University • How do we know there is a problem? • Why should we change? • Risks in preserving status quo • What should we do? • Priorities and longer term objectives

Research Integrity • • • Scientific quality Ethical integrity Financial and intellectual probity Safety and privacy of participants and researchers Compliance with legal and statutory requirements

Scope of Research Governance • • • Defining and setting standards Appropriate education and training Clear allocation of responsibilities and lines of accountability Transparent decision making processes Monitoring arrangements & regular review

Research Governance • Systems and processes that – work in tandem with other institutional processes – are complementary to other institutional aspirations – do not add unnecessary bureaucracy • Strong leadership

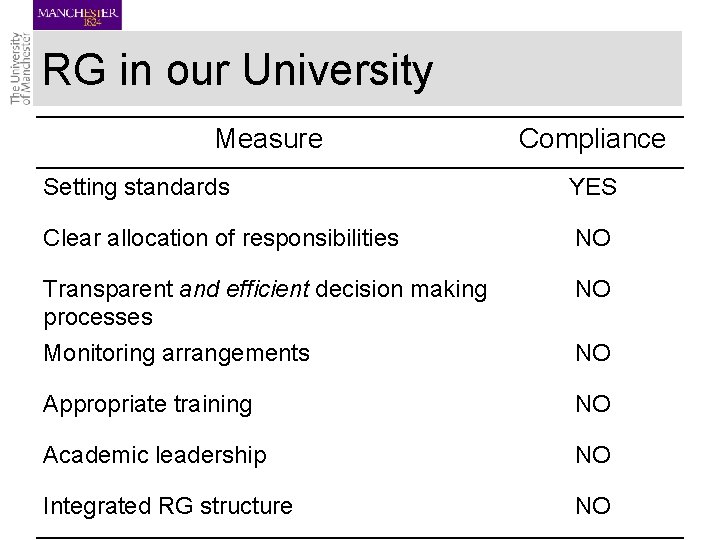
RG in our University Measure Compliance Setting standards YES Clear allocation of responsibilities NO Transparent and efficient decision making processes Monitoring arrangements NO Appropriate training NO Academic leadership NO Integrated RG structure NO NO

How do we know this is a problem? • Widespread ignorance of good practice and regulatory requirements • Poor research practices • Breaches of the regulatory requirements • “Known unknowns”

Why Should We Change? • Internal pressures – indications of a systemic problem – higher risk – unknown risk • External pressures – expectations of the public – regulatory requirements – requirements of research funders and partners • Right thing to do

Risks of preserving status quo • • • Harm/distress- subjects/researchers Breach of the law Reputation Reactive legislation/measures Financial penalty Poor quality/unpublishable research

What do we need to do? • Promote and support the good conduct of research • Have in place robust, transparent, fair, proportionate and efficient mechanisms to – ensure good practice – address failures if they arise

Basis for change • Manage systems not symptoms • Professionalism vs. ‘managerialism’ • Quality management

What do we need to do? • Shift emphasis from compliance to research integrity • Create academic-led research structure with clear lines of accountability • Have specific standards, policies and SOPs • Have a standard and efficient research approval process • Provide relevant training • Have risk-based monitoring • Have a robust research ethics review structure

Priorities • Research Conduct and Accountability Committee – Policy and decision making body • Regulatory compliance – MHRA • Person Responsible • Clinical Trials Management Group – HTA • Survey to determine holdings • HTA Research Licence Management Group • Research Ethics Committees – – 5 Committees University-wide membership Lay membership Standard operating procedures including appeals process

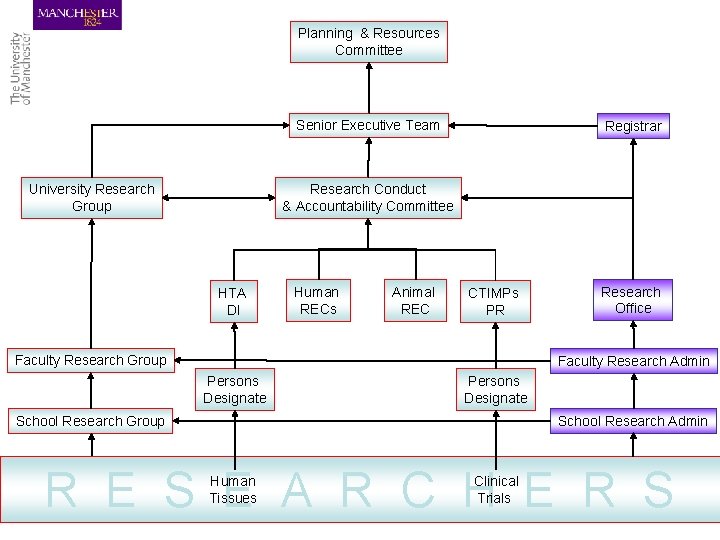
Planning & Resources Committee Senior Executive Team Registrar Research Conduct & Accountability Committee University Research Group HTA DI Human RECs Animal REC CTIMPs PR Faculty Research Group Research Office Faculty Research Admin Persons Designate School Research Group School Research Admin R E S E A R C H E R S Human Tissues Clinical Trials

Priorities • Research Conduct and Accountability Committee – Policy and decision making body • Regulatory compliance – MHRA • Person Responsible • Clinical Trials Management Group – HTA • Survey to determine holdings • HTA Research Licence Management Group • Research Ethics Committees – – 5 Committees University-wide membership Lay membership Standard operating procedures including appeals process

Medium and Long Term Objectives • New research approvals process • School Research Committees – – Management of research portfolio Research approval Training Monitoring • Changes introduced after – wide consultation – piloting of new procedures & processes – robust review

Final Thoughts…… • Act as we if intend to succeed but expect failure (‘optimism of the will and pessimism of the intellect’) • If we are going to fail, then let’s choose how we fail. • Let’s choose to fail wisely… whilst doing the right things.

Lessons from Toyota: From Ohno to Oh No! • Gold standard in production engineering • Based on – defining what needed to be done – working out better systems of doing it – rigorous methods for ensuring it’s done • Failure due to shift of focus from high production standards to being the biggest automotive manufacturer Taiichi Ohno

Lessons from Toyota • Any company (or institution), no matter how large and how famous for its merits, can stumble into making serious errors. • Pride and fear make it difficult to correct the error in good time. • Management decisions should normally never be taken on the basis of ‘profit’ alone.
 Scope of corporate governance
Scope of corporate governance Define tricker
Define tricker Scope management pmp
Scope management pmp Use case diagram
Use case diagram Quote one topic sentence
Quote one topic sentence Methodological integrity in qualitative research
Methodological integrity in qualitative research Uq research integrity module
Uq research integrity module Epigeum research integrity training
Epigeum research integrity training Scope definition in research
Scope definition in research Ipo model conceptual framework
Ipo model conceptual framework Iconic models in operations research
Iconic models in operations research Data collection plan research proposal
Data collection plan research proposal Scope of operations research
Scope of operations research Operations research topics
Operations research topics Scope of operation research
Scope of operation research Topical scope in research
Topical scope in research Developing a global vision through marketing research
Developing a global vision through marketing research Topical scope in research
Topical scope in research Scope of the study example
Scope of the study example Research problem meaning
Research problem meaning