The Grant Writing and Review Process at NIH


















![Main Sections of the R 01: Specific Research Plan n [Introduction – revisions only] Main Sections of the R 01: Specific Research Plan n [Introduction – revisions only]](https://slidetodoc.com/presentation_image_h2/3b6c3c15e25d5ed1c7adddbe7b424235/image-22.jpg)





































- Slides: 64

The Grant Writing and Review Process at NIH Joshua Smyth Professor of Biobehavioral Health and Medicine; Associate Director, SSRI Rhonda Be. Lue Associate Professor of Health Policy and Administration Jenae Neiderhiser Professor of Psychology Danielle Downs Associate Professor of Kinesiology and Obstetrics & Gynecology

Thanks to: n Lori Francis, Associate Professor of Biobehavioral Health n Brittany Frost, Social Science Research Institute

Workshop Outline n n NIH Organization NIH Funding Mechanisms The Grant Writing Process n Focus on the R 01 The NIH Review Process n n Overview of Review Meeting The Scoring Process A Penn State example Workshop Evaluation

I. The NIH § Department of Health and Human Services § National Institutes of Health § 25 Awarding Institutes/Centers aka ICs § § § e. g. , NICHD, NIMH, NIDA, NIA, etc. Center for Scientific Review Office of the Director

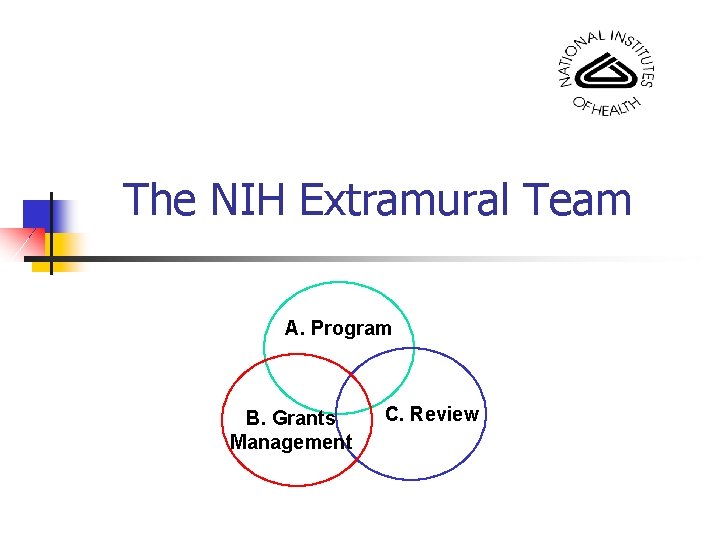
The NIH Extramural Team A. Program B. Grants Management C. Review

A. The Program/Institute Staff Program Administrator n n n n Maintains knowledge of scientific area Attends study section meetings Makes funding recommendations Monitors scientific progress Identifies scientific area of importance Reports to senior staff Development of programs and initiatives

B. Grants Management § § Interprets Federal regulations and policies Assures compliance with Federal regulations and policies Monitors financial aspects of projects Interprets regulations and policy

C. Review: Scientific Review Group (1 st Level) § § Center for Scientific Review (CSR) or NIH Institute & Center (IC) Scientific Review Group (SRG) § § Non-federal scientists with relevant expertise Led by a Scientific Review Officer (SRO) § http: //www. csr. nih. gov/Roster_proto/section. I. asp

C. Review: Advisory Council or Board (2 nd Level) n n n The potential awarding IC performs the second level of review NIH program staff examine applications for impact (formerly “priority”) scores, percentile rankings, & summary statements against the IC’s needs Program staff provide grant funding plan to Advisory Council or Board advises the IC director Director makes final decision

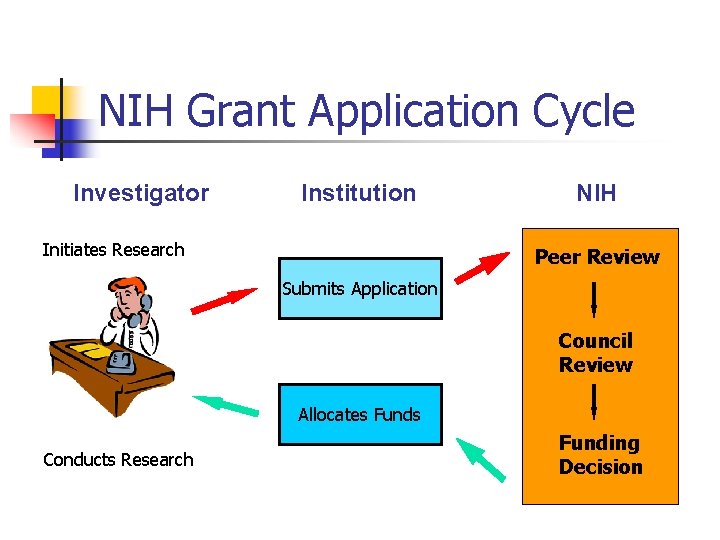
NIH Grant Application Cycle Investigator Institution Initiates Research NIH Peer Review Submits Application Council Review Allocates Funds Conducts Research Funding Decision

Grant Application: It’s a process, not an event 1. Communicate with Program Officer § Introducing ideas, getting feedback, pre-review 2. Get your proposal to the right review committee § § § Review the rosters and talk to colleagues Effectively wording the abstract Make a written request 3. Seek feedback from colleagues and consultants on drafts of the grant (prepare ahead!) 4. Consider who is likely to review your grant (review the rosters) and make sure to know and cite their work when relevant 5. Recognize that funding on first submission is rare

II. NIH Grant Mechanisms n n n n Ks: NIH Career Development Awards (K 01, K 02, K 05, K 07, K 08, K 22 [K 99/R 00]) P 01: Research Program Project Grant P 30: Center Core Grants R 01: NIH Research Project Grant Program R 03: NIH Small Grant Program R 13: NIH Support for Conferences and Scientific Meetings (R 13, U 13) R 15: NIH Academic Research Enhancement Award (AREA)

NIH Grant Mechanisms (continued) n n n R 21: NIH Exploratory/Developmental Research Grant Award R 34: NIH Clinical Trial Planning Grant T series: NRSA Training Grants (T 32, T 34, T 35, T 90, etc. ) U series: Research Project Cooperative Agreement Diversity Supplements: Research Supplements to Promote Diversity in Health-related Research Roadmap: NIH Roadmap Initiatives (Director’s Pioneer Award; Director’s New Innovative Program)

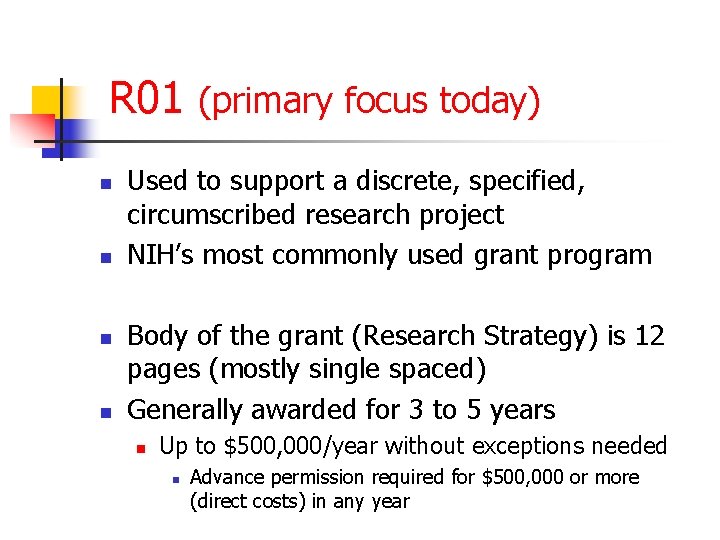
R 01 (primary focus today) n n Used to support a discrete, specified, circumscribed research project NIH’s most commonly used grant program Body of the grant (Research Strategy) is 12 pages (mostly single spaced) Generally awarded for 3 to 5 years n Up to $500, 000/year without exceptions needed n Advance permission required for $500, 000 or more (direct costs) in any year

New and Early Stage Investigators: A Competitive Edge n n New Investigator has not previously served as a PI for an R 01; may have been an investigator or received other smaller, developmental or research training awards Early Stage Investigator (ESI) is within 10 years of completing her/his terminal research degree, or completing medical residency

III. The Grant Writing Process n Grant writing is: n n n A skill like any other… But not the same skill as article writing Instead, more of a problem-based writing activity (theory and practice problem)

A few preliminary tips n Start early, make a timeline and STICK TO IT n n n Should allow time for serious pre-submission review and subsequent revision Develop a relationship with project officers It is not possible to overdo clarity Let your passion come through in your proposal Take advantage of early stage and new investigator opportunities

Getting ready to write n n Know what has been done Know what has been funded n n NIH website Re. PORTER (formerly CRISP) Decide on the problem n Important enough to get funded but simple enough to explain as clean design n n in 12 pp for the R 01; less for some other mechanisms Assemble team n Complementary skills; Seniority/competence/other by association; People you can count on

Getting ready to write n Communicate with program officer n n n Establish a relationship and trust (funding decision) Acquire information on mechanism and priorities Obtain input on aims/proposal

Main Sections of the NIH Application (see Francis et al. for some examples) n n Face Page Table of Contents Performance Sites Other information n Project Summary (Description) Public Health Relevance Statement Facilities & Resources

More Sections n Key Personnel n n Budgets (for each study year) n n Biosketches -- with personal statements Budget Justification Other sections (not discussed today); for example: n n Clinical Trial and Human Embryonic Stem Cell (HESC) List of Research Plan Attachments
![Main Sections of the R 01 Specific Research Plan n Introduction revisions only Main Sections of the R 01: Specific Research Plan n [Introduction – revisions only]](https://slidetodoc.com/presentation_image_h2/3b6c3c15e25d5ed1c7adddbe7b424235/image-22.jpg)
Main Sections of the R 01: Specific Research Plan n [Introduction – revisions only] Specific Aims: The basis for the proposal’s organization Research Strategy n n Significance and Innovation Approach n Preliminary studies n Design n Sample/recruitment/power analyses n Procedures & measures n Analyses

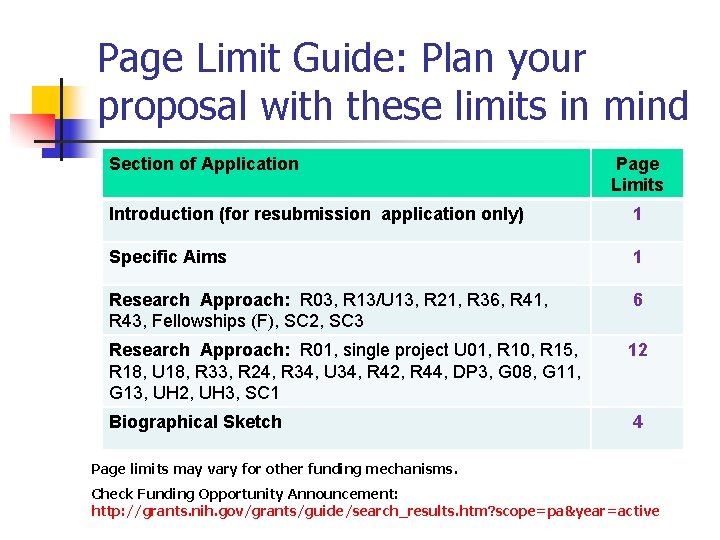
Page Limit Guide: Plan your proposal with these limits in mind Section of Application Page Limits Introduction (for resubmission application only) 1 Specific Aims 1 Research Approach: R 03, R 13/U 13, R 21, R 36, R 41, R 43, Fellowships (F), SC 2, SC 3 6 Research Approach: R 01, single project U 01, R 10, R 15, R 18, U 18, R 33, R 24, R 34, U 34, R 42, R 44, DP 3, G 08, G 11, G 13, UH 2, UH 3, SC 1 12 Biographical Sketch 4 Page limits may vary for other funding mechanisms. Check Funding Opportunity Announcement: http: //grants. nih. gov/grants/guide/search_results. htm? scope=pa&year=active

And More Sections n Protection of Human Subjects n n n n Women and Minorities Planned Enrollment Table Children References Cited Letters of Support Resource Sharing Plan Checklist SO START EARLY!

General themes of success n Clarity is key – each point follows naturally n n Tell a (scientific) story Everything that needs to be there, is; nothing extra Communicate your excitement Get feedback early on n And often

General themes to R 01 Success n Impact and Significance n n Practice (2 -3 sentences) n Prevalence of problem in population n Important social concern/public health problem Theory (model) n n Innovation n n Building, testing, using New directions, value added Compelling Preliminary Research

Keys to Success – Specific Aims n Specific Aims may be only part read by some reviewers n n No more than one page Tell the entire story n n n Functions as an abstract would in a manuscript End with (actual) specific aims Hypotheses and aims must align

Keys to Success – Background n Background and significance n n This is where you ‘hook’ the reader on your story n n Used to be an explicit section; no longer Functions as the ‘introduction’ does in a manuscript Likely no more than 2 -3 pages

Preliminary Studies n Research team collaboration no longer required to be documented n n Still very helpful Preliminary study ‘types’ – document: n n Relevant previous research Research that demonstrates competence in requisite domains Capacity to recruit in specific populations and/or contexts Pilot data specific to proposal

Keys to Success - Methods n Methods are very important n n n Overall -- clarity and detail May include a table that traces aims to hypotheses to constructs to measures (table/s) Is the design feasible? Are there gaps in the methods (e. g. , fidelity for interventions) Statistics are essential – product must match aims n n Consider a methodologist team member Include a detailed timeline

And More Methods: The Sample n Preference for representative samples n n Generalizability from a single entity (university, clinic, state) n n n Students only if relevant to age/situation (e. g. , college drinking) Justified exclusions Unit of assignment is unit of analysis Sample size/power analysis n For each outcome/planned test

Budgets n Direct Costs n n n Senior Personnel (PI, co-Is, project director) (PSU fringe at 36. 5%) Other Personnel (full time staff, RAs, part time wages) (PSU fringe 36. 5% for full time staff, 13. 2% for Grad Assts AY; 7. 9% for part time wages and summer) Equipment Travel Participant/Trainee Support Costs

Budgets, cont. n Other n n n n Materials and supplies Publication costs Consultant services Subawards/Consortium/Contractual Fees Other Indirect Costs (~50% at PSU, but does not include all expenses) Budget justification

Receiving the Summary Statements: The Hardest Part! 1. Reviews critical, even harsh 2. Reviewers usually find grant’s weaknesses, while recognizing strengths 3. Summary statements spend much more time on critique than praise 4. Many investigators experience a mixture of rage and depression when they read their summary statements and easily lose perspective 5. Take a day or two (or more!) and then read again with a cooler head

Receiving the Summary Statements: Bouncing Back! 1. Ask experienced colleagues to read reviews 2. Don’t interpret criticism as hopeless 3. Program Officer may be helpful in clarifying critique 4. If “discussed” (rather than triaged), you have a chance of funding in next round 5. The lower the initial score, the fewer problems and more likely to be successful after revision

Resubmission: Resilience and Flexibility! 1. Persistence pays off in the grant process!! 2. Second submission must respond to the critiques through revision or clearly defending reasoning 3. Same reviewers may or may not review resubmission, but will see critique

Most Common Reasons for a Poor Score (in priority order) n n n n Lack of impact or significance Lack of new or original ideas Hypotheses ill-defined, superficial, lacking, unfocused, or unsupported by preliminary data Methods unsuitable, not feasible, not rigorous or not likely to yield results; methods don’t clearly link to aims Design not logical, inappropriate instrumentation, poor timing or conditions; doesn’t link well to aims Data management and analysis vague, not rigorous; analyses don’t clearly link to aims Inadequate expertise or knowledge of field for PI; too little time to devote to the work Poor resources or facilities; limited access to appropriate population

When to Revise n n Basic idea was significant and innovative or these can be bolstered Design/measurement/analysis problems can be clarified (more information) or fixed Need preliminary data Problem is poor writing

IV. The NIH Review Process § § A. The Review Meeting B. Review Discussion C. The Scoring Process D. A Penn State Example

A. The Review Meeting: The SRO’s Role Prior to Meeting § § § Point of contact until review group meets (then project officer) Analyze submissions for completeness and conflicts Recruit ad hoc reviewers as needed Schedule 1 -2 day meeting Assign applications to reviewers (at least 3) § § Primary, secondary, discussant Create review order based on preliminary impact scores from best to worst within categories

Reviewers’ Role Prior to Meeting § § Familiarize self with criteria, mechanisms, and scoring Review assigned applications § § § § Assign scores to each criteria and other areas Write bulleted strengths and weaknesses for each criteria Reviews are advice to institutes for funding decisions, not advice to PI Post scores and comments on NIH Commons Read other reviews of assigned applications Prepare presentation of reviews Skim/read non-assigned applications

Format of the Review Meeting n n n SRO opening remarks Chair orientation New investigator R 01 grants Other grant types (R 03, R 15, R 21, R 34) n Applications discussed in order of Impact Score; bottom 50% are not discussed

SRO Opening Remarks n n n Confidentiality Review order Proposals below median within each category may not be discussed

Chair Orientation § Start with reviewer impact scores § § § May differ from posted scores Goal of discussion is to clarify not reach agreement If scores are similar, shorter discussion If scores are dissimilar, longer discussion Recommended time § § § Primary – 5 minutes Secondary – 3 minutes Discussant – 2 minutes

B. Review Discussion n n Identify proposal Members in conflict leave Reviewers provide preliminary impact scores Reviews n n n Impact, Significance, Investigators, Innovation, Approach, Environment Stress main points, do not repeat previous points Non-reviewers typically ask questions to clarify Human Subjects issues affecting scoring Open discussion to entire committee

Review Discussion (continued) n n n Ask for reviewers impact scores again Identify the reviewers’ recommended range Ask if anyone wants to score outside the range Entire committee records impact score Discuss budget and other issues

C. The Scoring Process 1. Overall Impact Score: likelihood project will “exert a sustained, powerful influence on the research field(s) involved (1 -9 scale) 2. A separate 1 -9 score for each of 5 core criteria (Significance, Investigators, Innovation, Approach, Environment) 3. Additional review criteria help determine scientific and technical merit BUT are not scored separately 4. Additional review considerations are addressed by reviewers, but are not scored & are discussed after group scores

Score Criteria n Overall Impact: will project exert a sustained, powerful influence on the research field(s) as indexed by 5 core review criteria 1. Significance: important problem addressed; how will this improve scientific knowledge, technical capability, and/or clinical practice

What is the Difference Between Impact and Significance ? n Impact Addresses: n n Probability of whether the research will exert a sustained, powerful influence on the research field Significance Addresses: n n Does the project address an important problem or a critical barrier to progress in the field? If the aims are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved?

Score Criteria (continued) 2. Investigators: PI & other researchers well suited to the project; appropriate experience & training; ongoing record of accomplishments; complementary & integrated experience; leadership approach, governance, and organizational structure appropriate for project 3. Innovation: the work challenges and seeks to shift current research or practice paradigms; utilizing novel theory, approaches or methods, instrumentation, or interventions; the work is novel (Be innovative, but maybe not too innovative…)

Score Criteria (continued) 4. Approach: strategy, methodology, analyses are wellreasoned and appropriate; potential problems & alternative strategies thought through; benchmarks set; risk is managed Most common reviewer complaint is lack of detail here Typically the longest section 5. Environment: the environment will contribute to the project’s success; institutional support, equipment, & other resources sufficient; unique features of the environment, subject population, collaborative arrangements

Additional Review Criteria (not scored) Human Subjects: 1. 2. 3. 4. 5. Protection of human subjects Data safety monitoring plan (clinical trials only) Inclusion of women, minorities, children Vertebrate animals Biohazards

Additional Review Considerations 1. 2. 3. 4. Budget and period of support Select agent research (infectious agents) Applications from foreign organizations Resource sharing plans

CRITERIA SCORING SYSTEM HIGH 1. Exceptional: Exceptionally strong with essentially no weaknesses 2. Outstanding: Extremely strong with negligible weaknesses 3. Excellent: Very strong with only some minor weaknesses MEDIUM 4. Very Good: Strong but with numerous minor weaknesses 5. Good: Strong but with at least one moderate weakness 6. Satisfactory: Some strengths but also some moderate weaknesses Low 7. Fair: Some strengths but with at least one major weakness 8. Marginal: A few strengths and a few major weaknesses 9. Poor: Very few strengths and numerous major weaknesses

CRITERIA SCORING SYSTEM (continued) n n n Minor Weakness: An easily addressable weakness that does not substantially lessen impact Moderate Weakness: A weakness that lessens impact Major Weakness: A weakness that severely limits impact

CRITERIA SCORING SYSTEM (continued) Final Overall Impact Score: Mean of all reviewers’ final impact scores X 10 Range = 10 (high impact) -- 90 (low impact) NOTE: Scoring likely to produce applications with identical scores (“ties”). Thus, other factors (e. g. , mission relevance, portfolio balance) will be considered when all other things are essentially equal

Key Sections in R 01 Proposal Format n Research Plan Components n n n Specific Aims Research Strategy n Includes Background & Significance; Preliminary Studies/Progress Report; Research Design & Methods Facilities and Equipment n Reflects the Environment criterion n n For ESIs should describe the institutional investment in the success of the investigator Biographical Sketch [NEW! as of Jan 25 th, 2015] n n Personal statement – why well-suited for project, 4 pubs Contribution to science - you describe up to 5 of your most significant scientific contributions (<=1/2 page each); up to 4 pubs or other products for each contribution area

D. A Penn State Example Mock NIH Study Section Chair: Joshua Smyth, Associate Director, SSRI Reviewer #1: Rhonda Be. Lue, Associate Professor of Health Policy and Administration Reviewer #2: Jenae Neiderhiser, Professor of Psychology Reviewer #3: Danielle Downs, Associate Professor of Kinesiology and Obstetrics & Gynecology Review group members: Workshop attendees

Links of Interest Enhancing Peer Review Criteria: http: //grants. nih. gov/grants/guide/notice-files/not-od-09025. html Page Limits: http: //grants. nih. gov/grants/forms_page_limits. htm Human Subjects: http: //grants. nih. gov/grants/policy/hs/index. htm SF 424 guidelines for submission: http: //grants. nih. gov/grants/funding/424/index. htm Glossary: http: //grants. nih. gov/grants/glossary. htm

General Resources NIH Re. Porter (formerly CRISP) https: //libraries. ucsd. edu/info/resources/nih-reporterformerly-crisp Revised Applications http: //grants. nih. gov/grants/policy/amendedapps. htm NIH Grant Writing Tip Sheets http: //grants. nih. gov/grants/grant_tips. htm Getting an RO 1 http: //sciencecareers. sciencemag. org/career_development/ previous_issues/articles/1190/getting_an_nih_r 01

More general resources NSF Proposal Writing http: //www. cs. cmu. edu/~sfinger/advice. html Other Proposal Writing Guides http: //www. learnerassociates. net/proposal/ Reasons Proposals Fail http: //chronicle. com/article/How-to-Fail-in-Grant. Writing/125620/--let

New and Early Stage Investigator Resources New and Early Stage Investigators http: //grants. nih. gov/grants/new_investigators/

NIH Websites http: //public. csr. nih. gov/Pages/default. aspx http: //grants. nih. gov/grants/oer. htm (forms, grant search, etc. )

SSRI Listserv n n New subscribers can join the SSRI listserv by sending mail to: L-SSRI-subscribe-request@lists. psu. edu