Stereochemistry refers to the 3 dimensional properties and



![Specific Rotation, [α] = α / cl a = observed rotation c = concentration Specific Rotation, [α] = α / cl a = observed rotation c = concentration](https://slidetodoc.com/presentation_image_h2/6a0354032aeeb5e4fd96e2f97bdf0060/image-9.jpg)















- Slides: 53

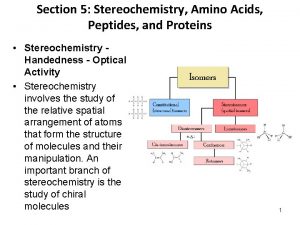
Stereochemistry refers to the 3 -dimensional properties and reactions of molecules. It has its own language and terms that need to be learned in order to fully communicate and understand the concepts.


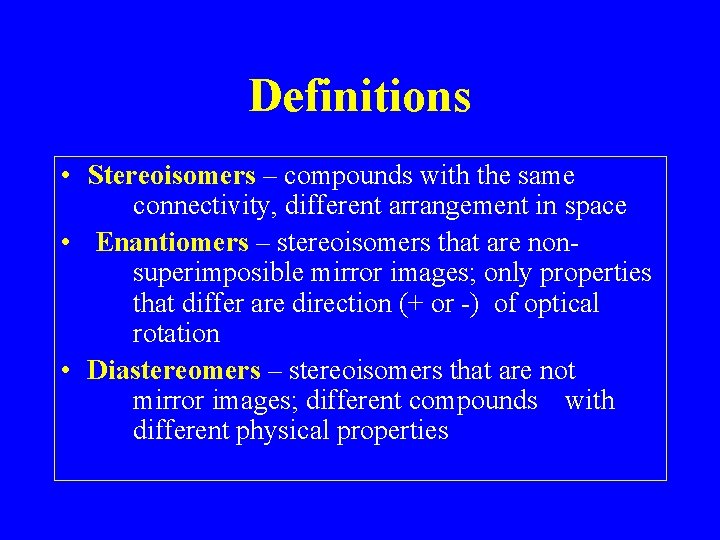
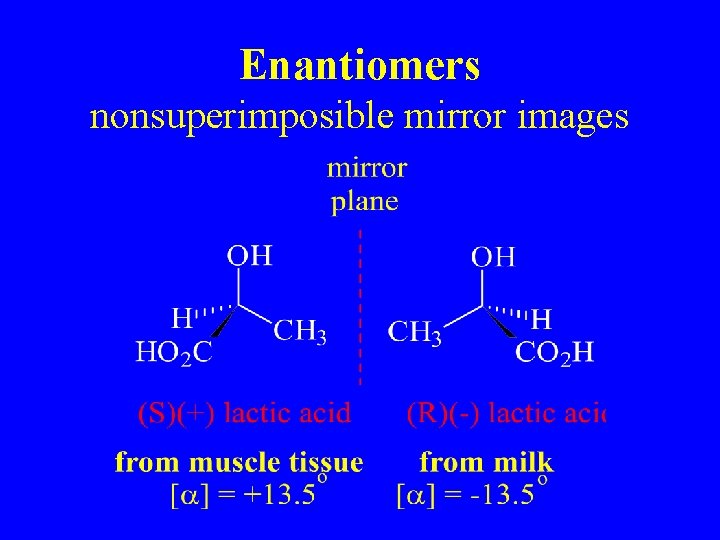
Definitions • Stereoisomers – compounds with the same connectivity, different arrangement in space • Enantiomers – stereoisomers that are nonsuperimposible mirror images; only properties that differ are direction (+ or -) of optical rotation • Diastereomers – stereoisomers that are not mirror images; different compounds with different physical properties

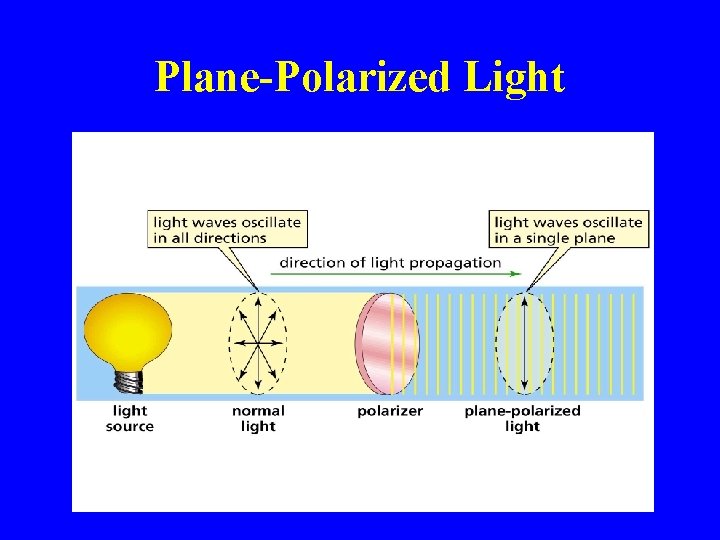
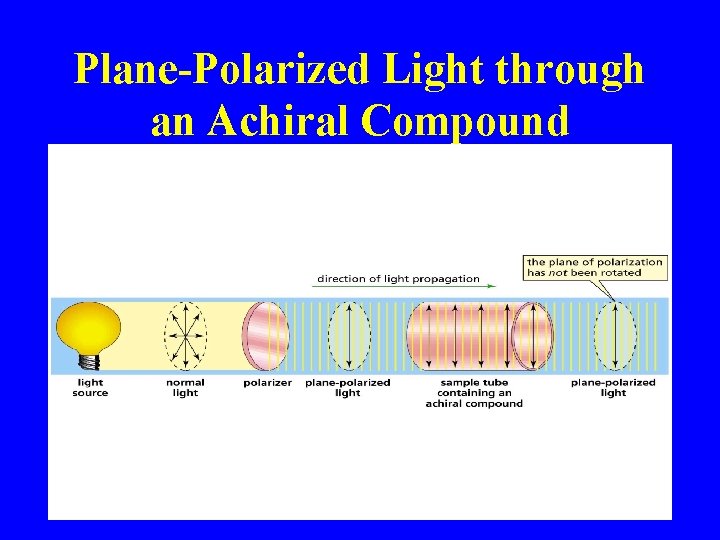
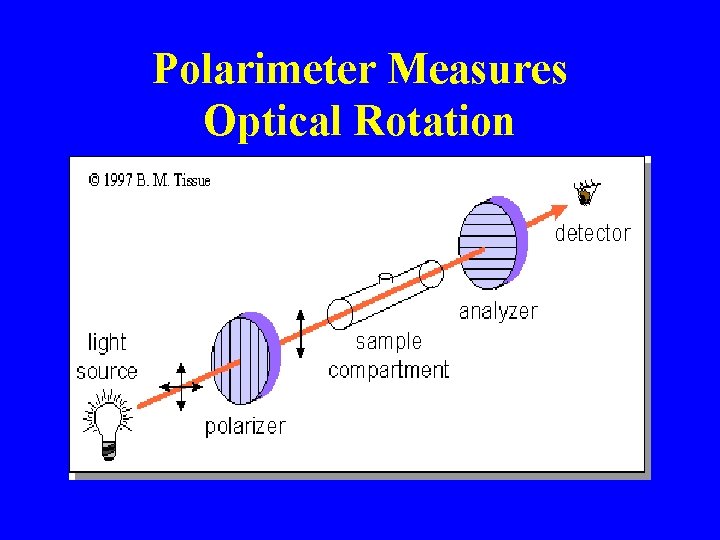
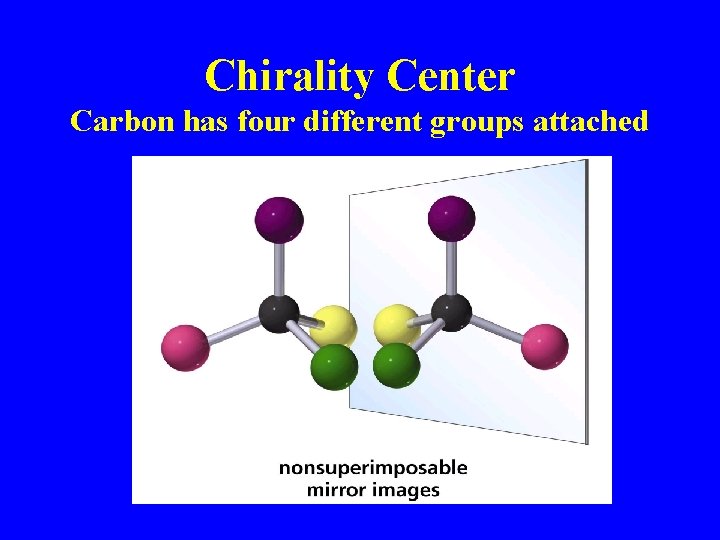
More Definitions • Asymmetric center – sp 3 carbon with 4 different groups attached • Optical activity – the ability to rotate the plane of plane –polarized light • Chiral compound – a compound that is optically active (achiral compound will not rotate light) • Polarimeter – device that measures the optical rotation of the chiral compound

Plane-Polarized Light

Plane-Polarized Light through an Achiral Compound

Plane-Polarized Light through a Chiral Compound

Polarimeter Measures Optical Rotation
![Specific Rotation α α cl a observed rotation c concentration Specific Rotation, [α] = α / cl a = observed rotation c = concentration](https://slidetodoc.com/presentation_image_h2/6a0354032aeeb5e4fd96e2f97bdf0060/image-9.jpg)
Specific Rotation, [α] = α / cl a = observed rotation c = concentration in g/m. L l = length of tube in dm Dextrorotary designated as d or (+), clockwise rotation Levorotary designated as l or (-), counterclockwise rotation

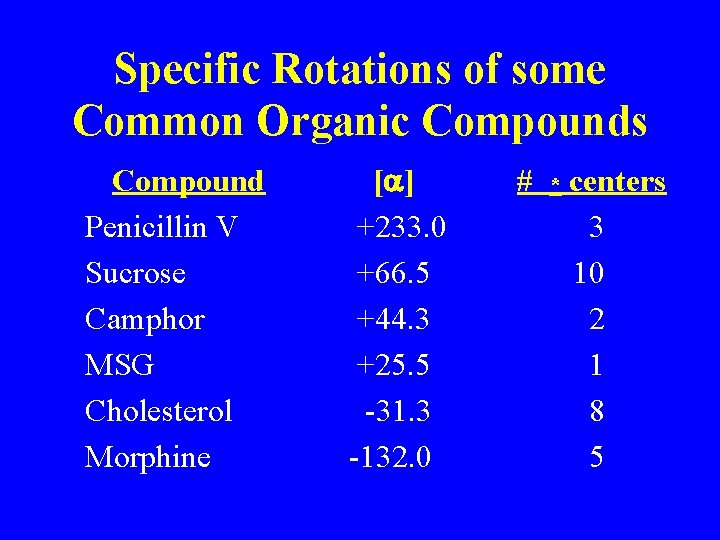
Specific Rotations of some Common Organic Compounds Compound Penicillin V Sucrose Camphor MSG Cholesterol Morphine [a] +233. 0 +66. 5 +44. 3 +25. 5 -31. 3 -132. 0 # * centers 3 10 2 1 8 5

Chirality Center Carbon has four different groups attached

Enantiomers nonsuperimposible mirror images

Enantiomeric Excess (Optical Purity)


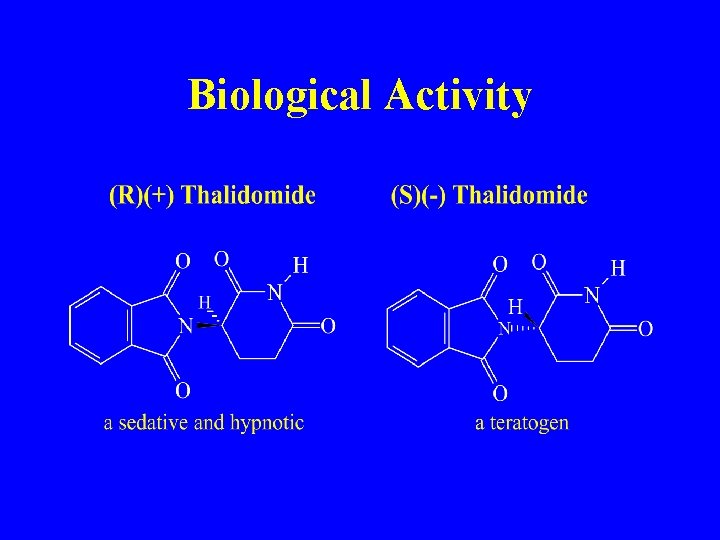
Biological Activity

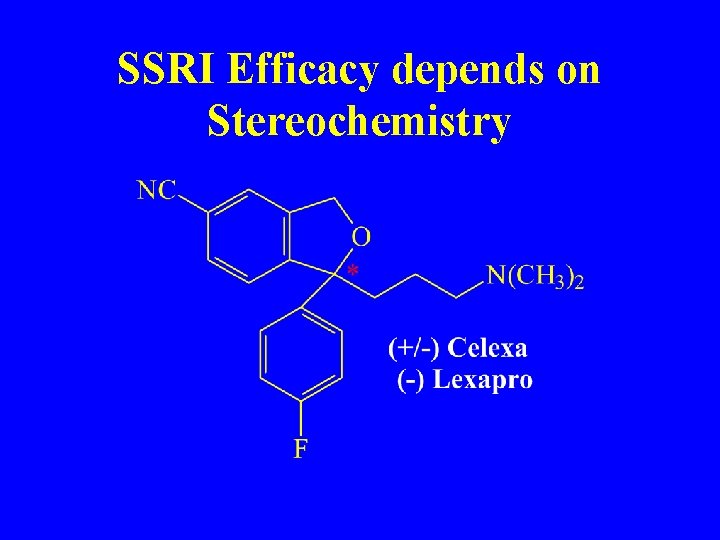
SSRI Efficacy depends on Stereochemistry

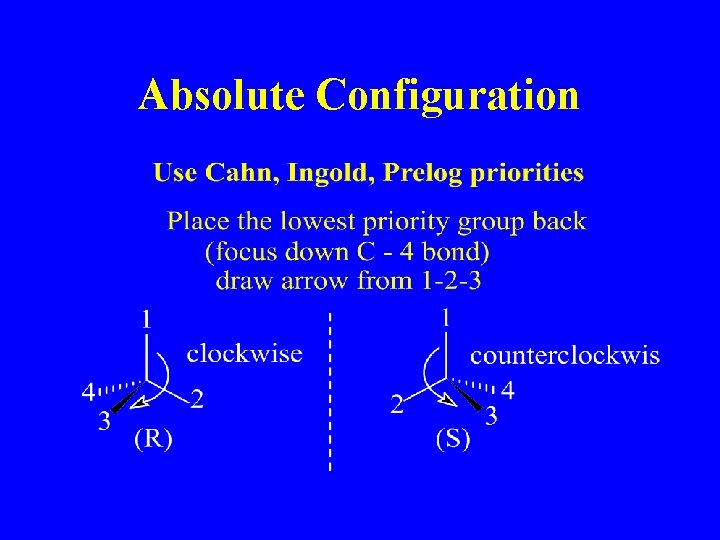
Absolute Configuration

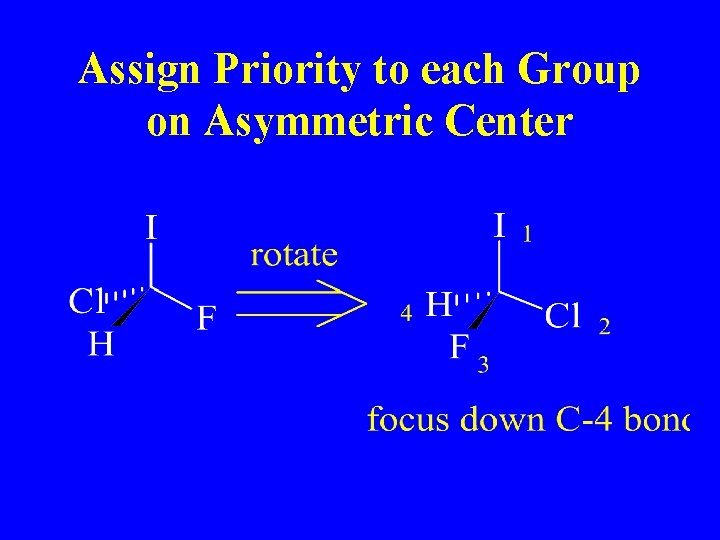
Assign Priority to each Group on Asymmetric Center

Lactic Acid

C. I. P. Priorities

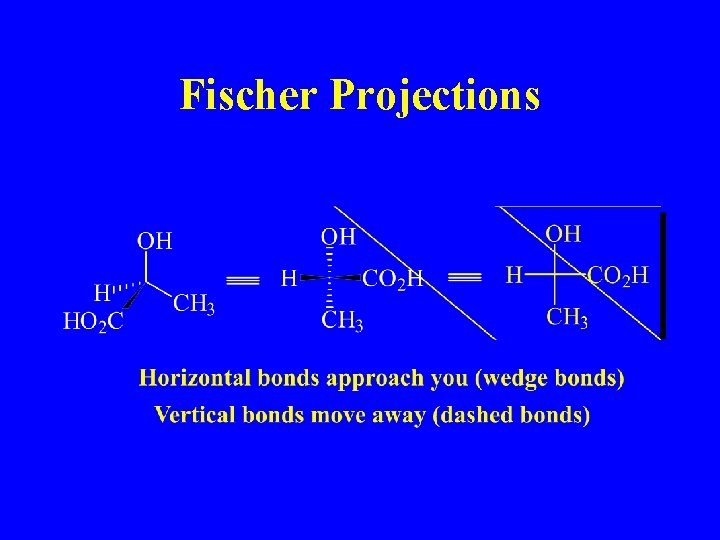
Fischer Projections

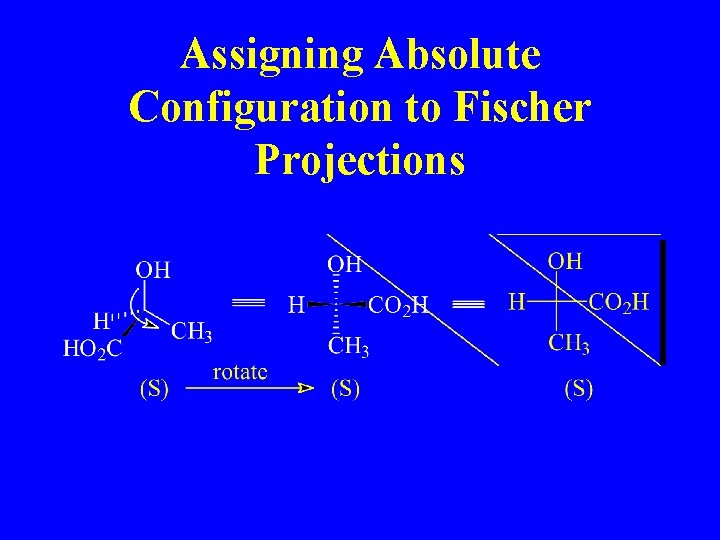
Assigning Absolute Configuration to Fischer Projections

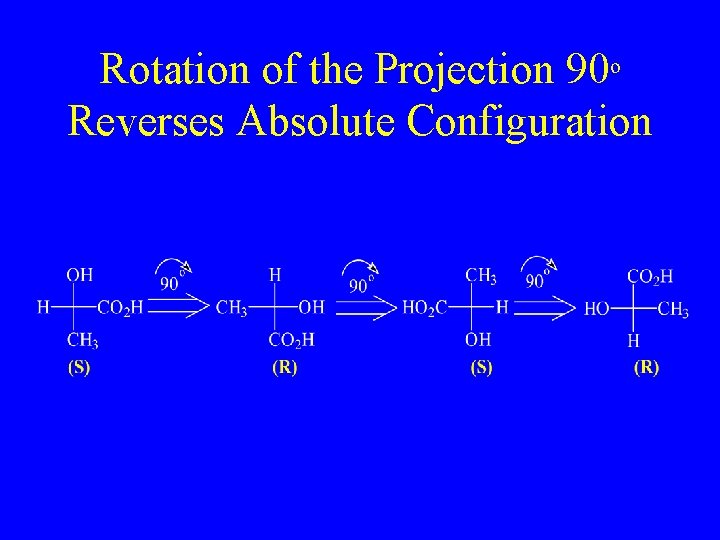
Rotation of the Projection 90 o Reverses Absolute Configuration

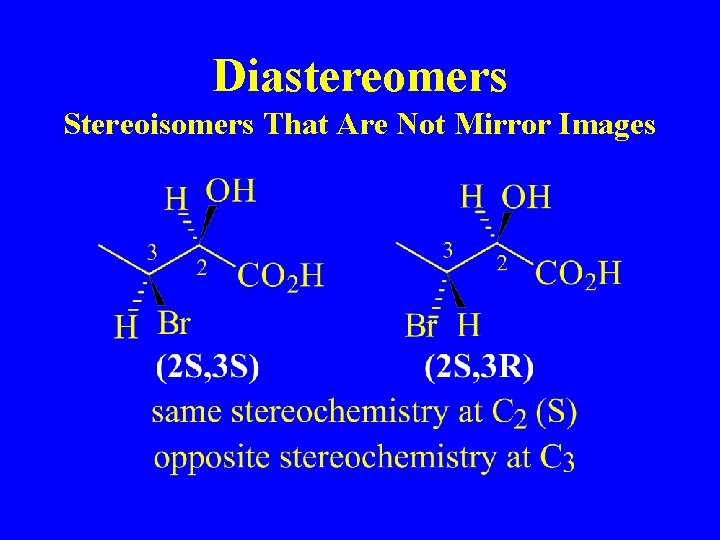
Diastereomers Stereoisomers That Are Not Mirror Images

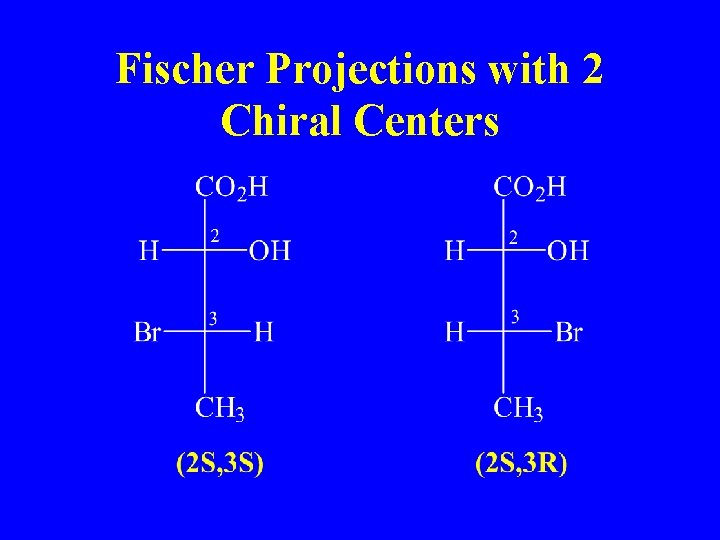
Fischer Projections with 2 Chiral Centers

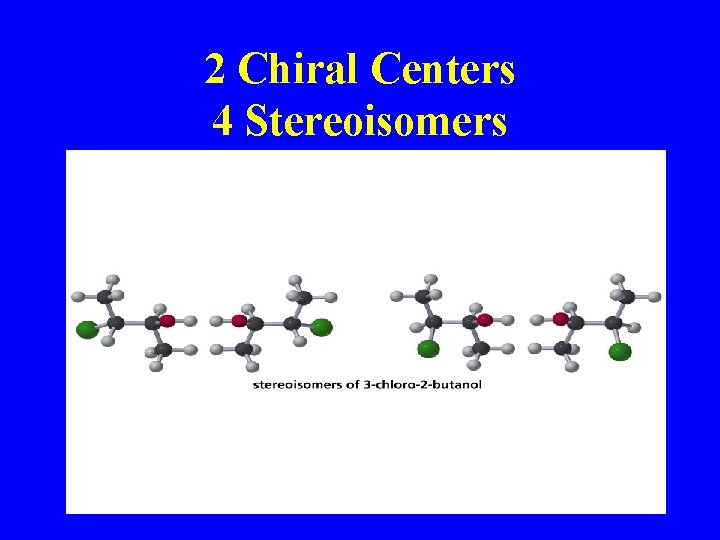
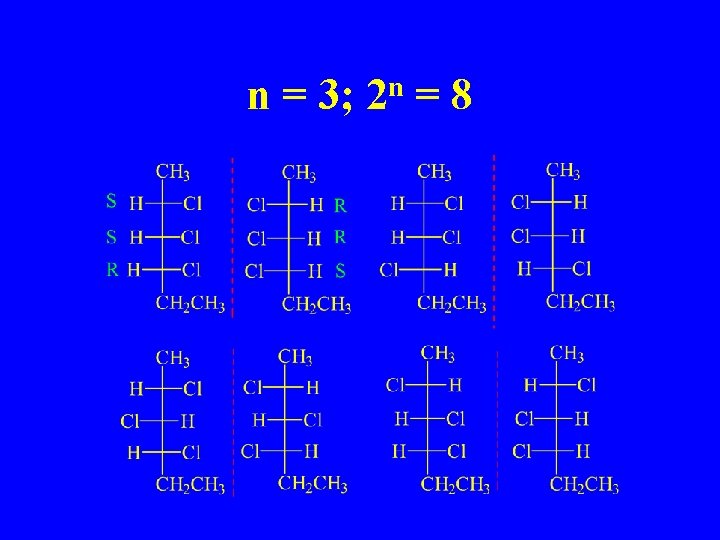
2 Chiral Centers 4 Stereoisomers

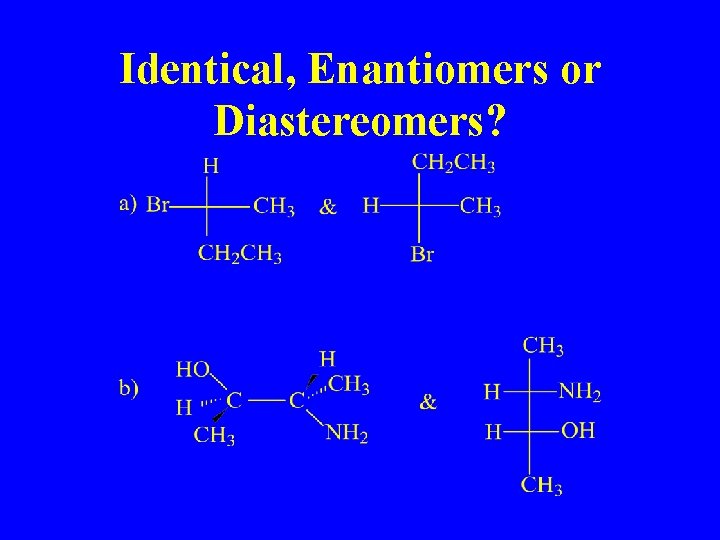
Identical, Enantiomers or Diastereomers?

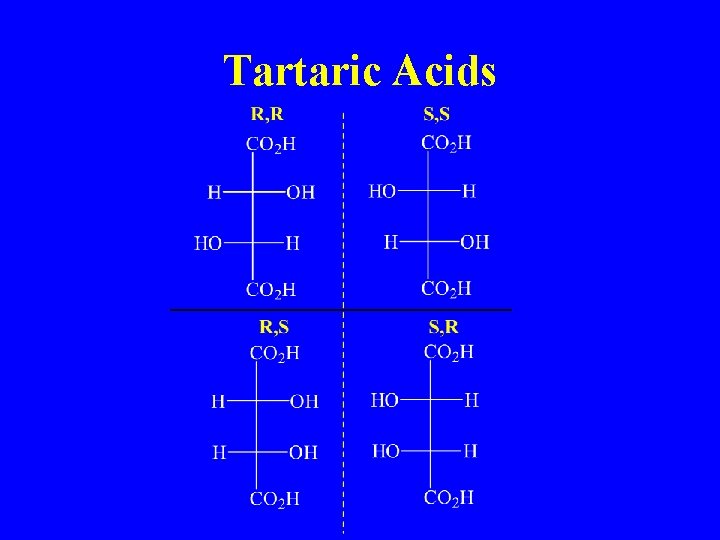
Tartaric Acids

Racemic Mixture

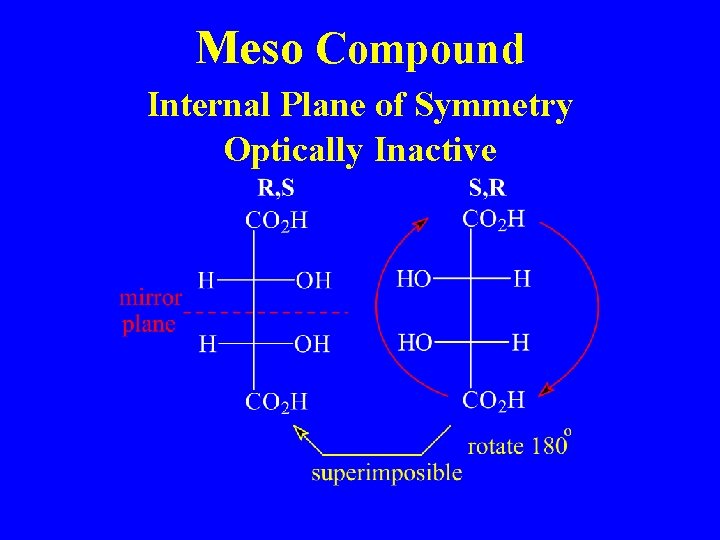
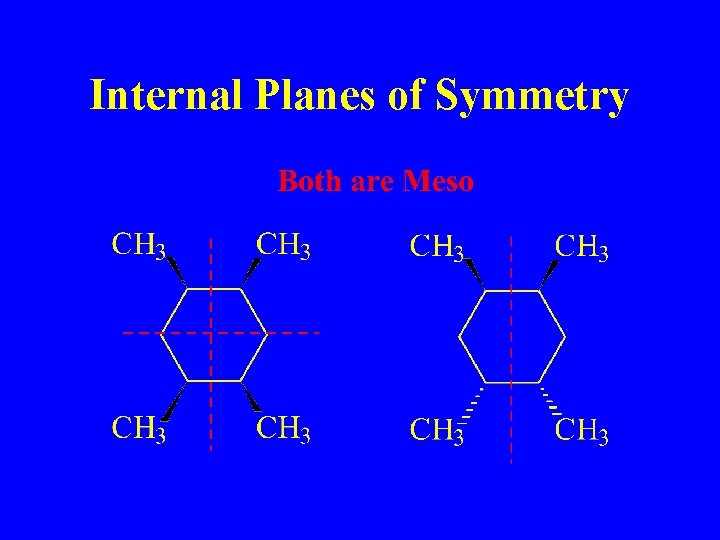
Meso Compound Internal Plane of Symmetry Optically Inactive

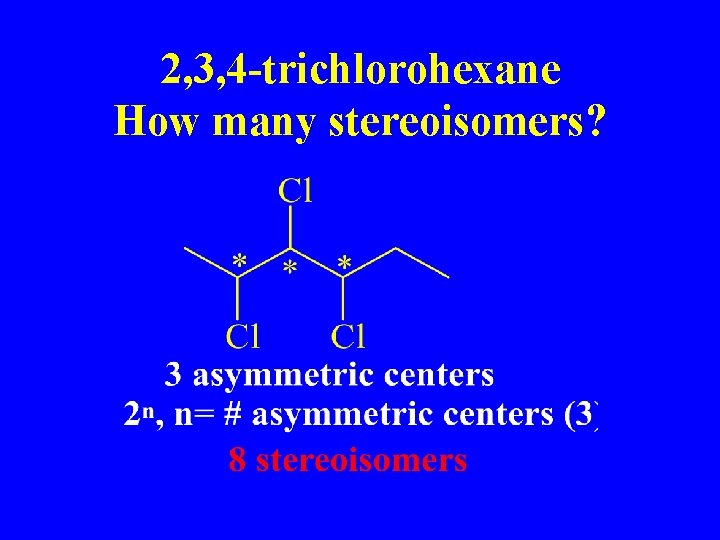
2, 3, 4 -trichlorohexane How many stereoisomers?

n = 3; n 2 =8

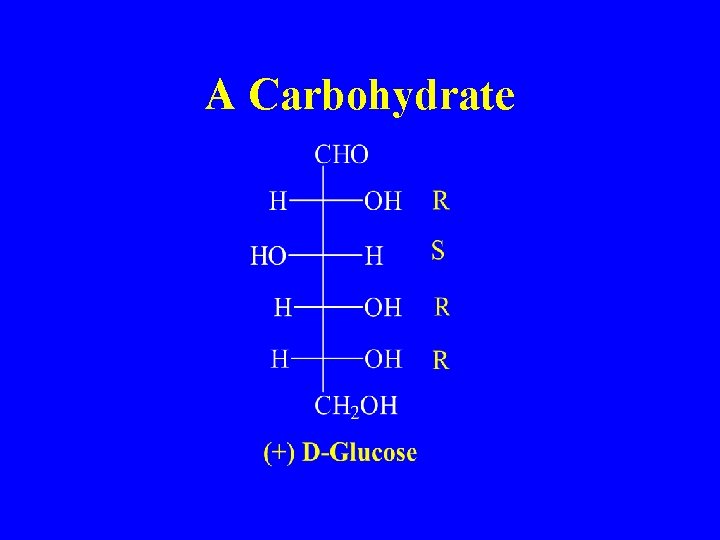
A Carbohydrate

Internal Planes of Symmetry

Asymmetric Centers on Rings

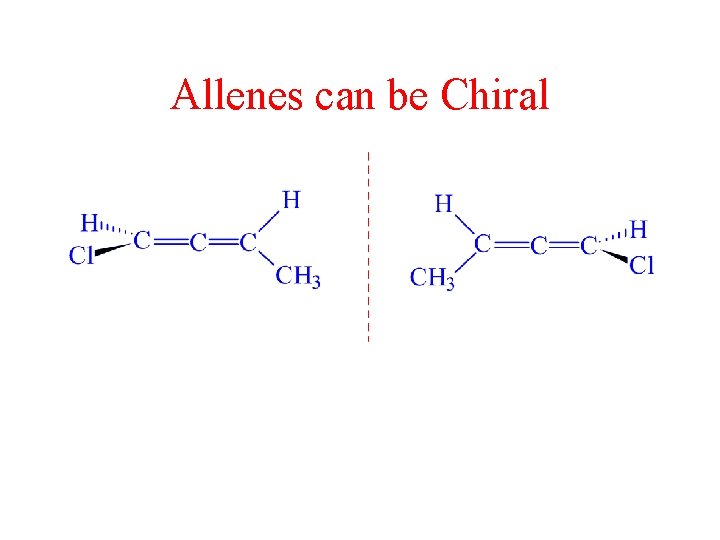
Allenes can be Chiral

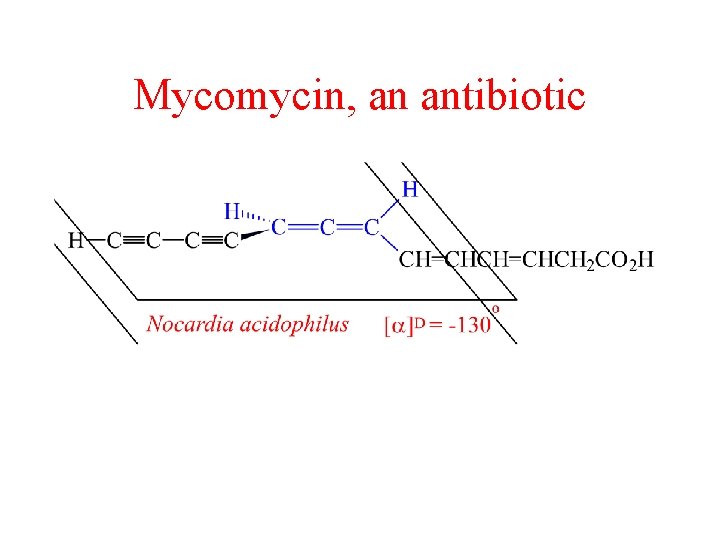
Mycomycin, an antibiotic

Reactions that Generate Chirality Centers Hydrogenation, syn

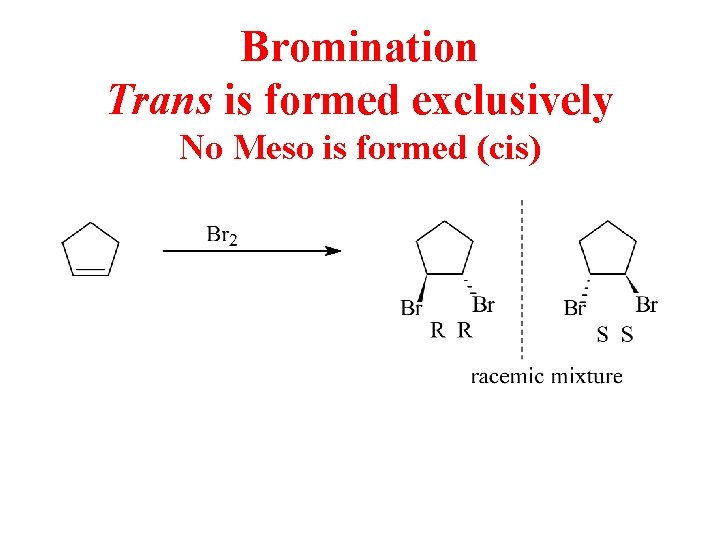
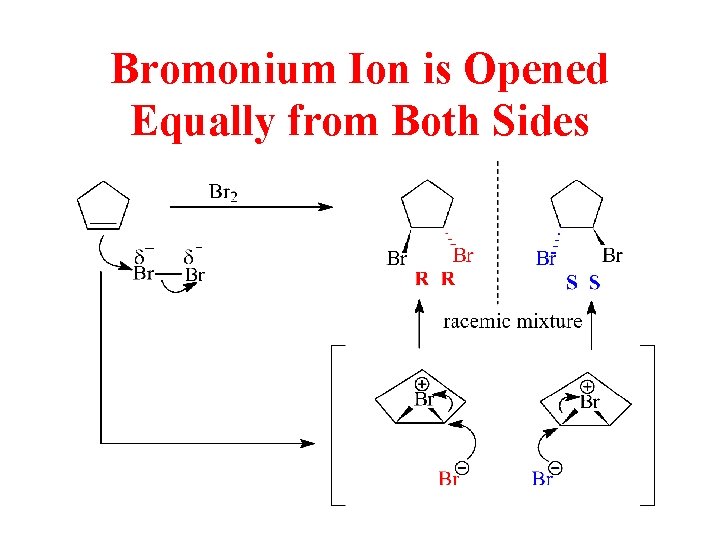
Bromination Trans is formed exclusively No Meso is formed (cis)

Bromonium Ion is Opened Equally from Both Sides

trans alkene + anti addition = MESO

cis Alkene + anti addition = racemic mixture

Brominations Often Generate Asymmetric Centers

Asymmetric Center is Generated Racemic Mixture Formed

Asymmetric Induction

Preparation of (L)-Dopa for Treatment of Parkinson’s

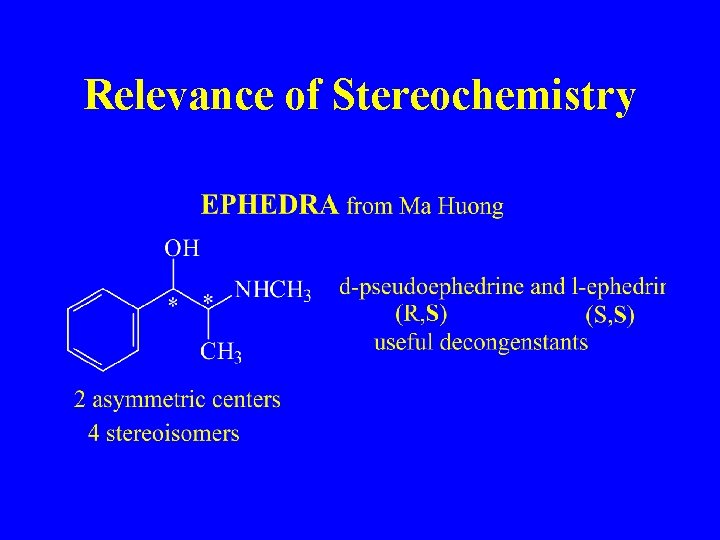
Relevance of Stereochemistry

One-step synthesis

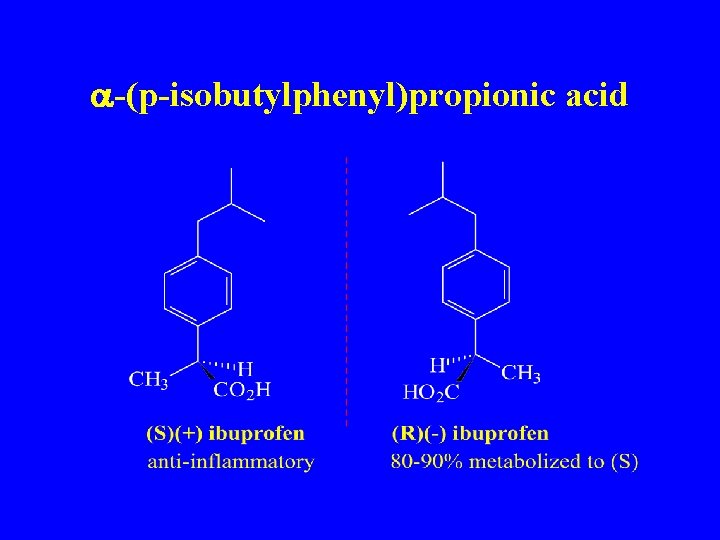
a-(p-isobutylphenyl)propionic acid

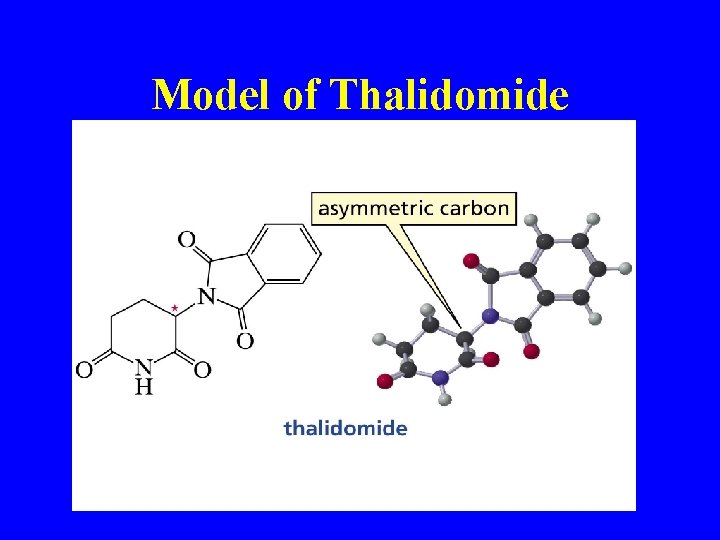
Model of Thalidomide

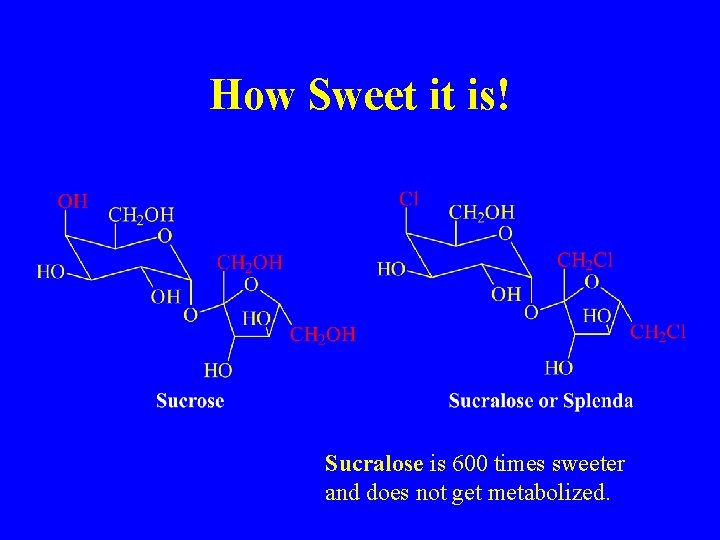
How Sweet it is! Sucralose is 600 times sweeter and does not get metabolized.

Sildenafil (Viagra) and Caffeine

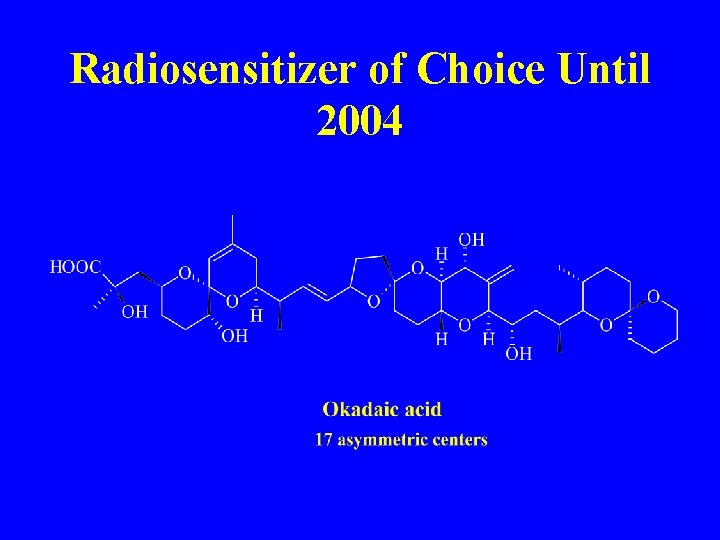
Radiosensitizer of Choice Until 2004
 Cyclopentane cis trans isomers
Cyclopentane cis trans isomers Ethane
Ethane A circular motion is one dimensional
A circular motion is one dimensional Peptide stereochemistry
Peptide stereochemistry Stereoisomers always possess handedness.
Stereoisomers always possess handedness. Stereochemistry quiz
Stereochemistry quiz Father of stereochemistry
Father of stereochemistry Introduction to stereochemistry
Introduction to stereochemistry Naproxen stereochemistry
Naproxen stereochemistry Stereochemistry of cephalosporin
Stereochemistry of cephalosporin Introduction to stereochemistry
Introduction to stereochemistry Properties of cube and cuboid
Properties of cube and cuboid Extensive properties and intensive properties
Extensive properties and intensive properties Chemical and physical properties
Chemical and physical properties Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là So nguyen to
So nguyen to Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Sơ đồ cơ thể người
Sơ đồ cơ thể người