Chapter 7 STEREOCHEMISTRY Stereochemistry CHIRALITY AND ENATIOMERS 1

- Slides: 9

Chapter 7 STEREOCHEMISTRY Stereochemistry

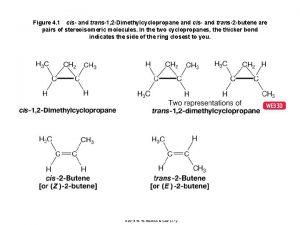
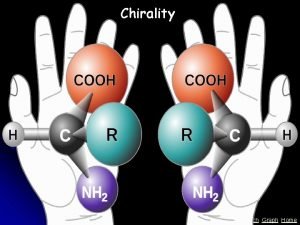
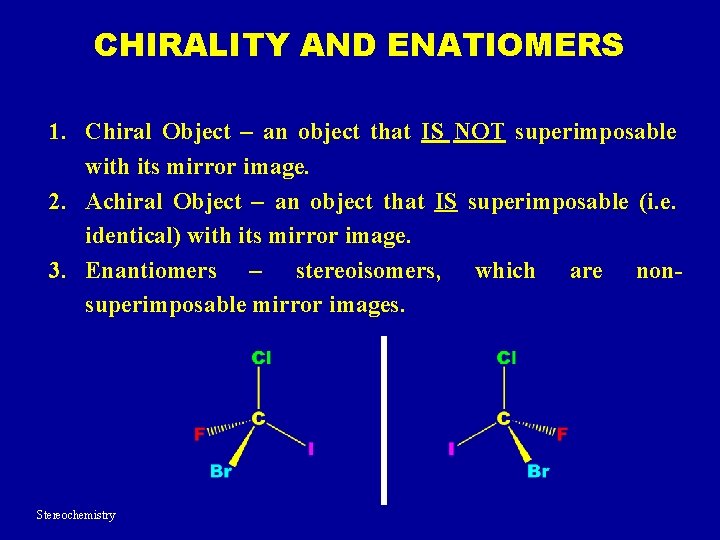
CHIRALITY AND ENATIOMERS 1. Chiral Object – an object that IS NOT superimposable with its mirror image. 2. Achiral Object – an object that IS superimposable (i. e. identical) with its mirror image. 3. Enantiomers – stereoisomers, which are nonsuperimposable mirror images. Stereochemistry

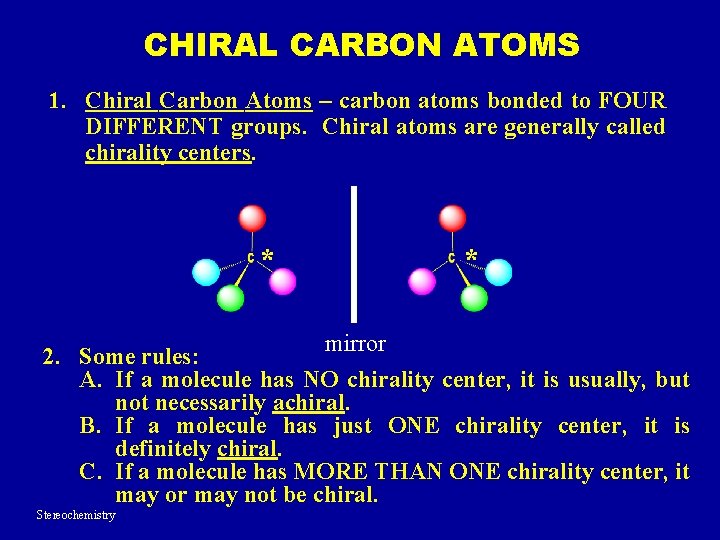
CHIRAL CARBON ATOMS 1. Chiral Carbon Atoms – carbon atoms bonded to FOUR DIFFERENT groups. Chiral atoms are generally called chirality centers. * * mirror 2. Some rules: A. If a molecule has NO chirality center, it is usually, but not necessarily achiral. B. If a molecule has just ONE chirality center, it is definitely chiral. C. If a molecule has MORE THAN ONE chirality center, it may or may not be chiral. Stereochemistry

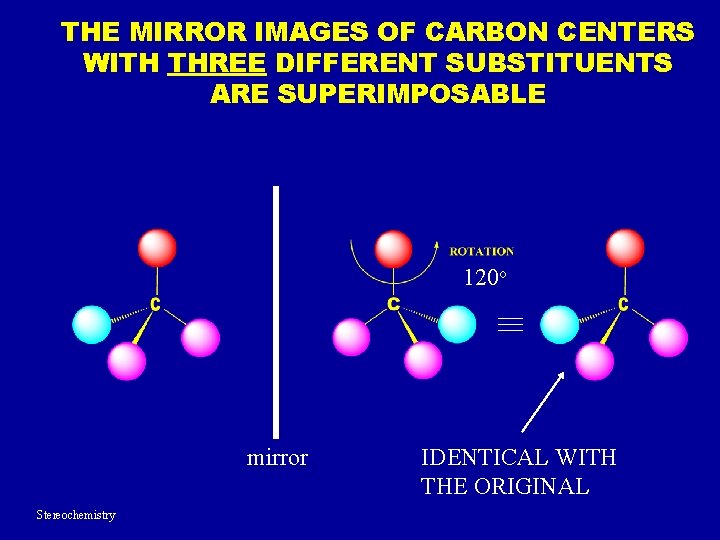
THE MIRROR IMAGES OF CARBON CENTERS WITH THREE DIFFERENT SUBSTITUENTS ARE SUPERIMPOSABLE 120 o mirror Stereochemistry IDENTICAL WITH THE ORIGINAL

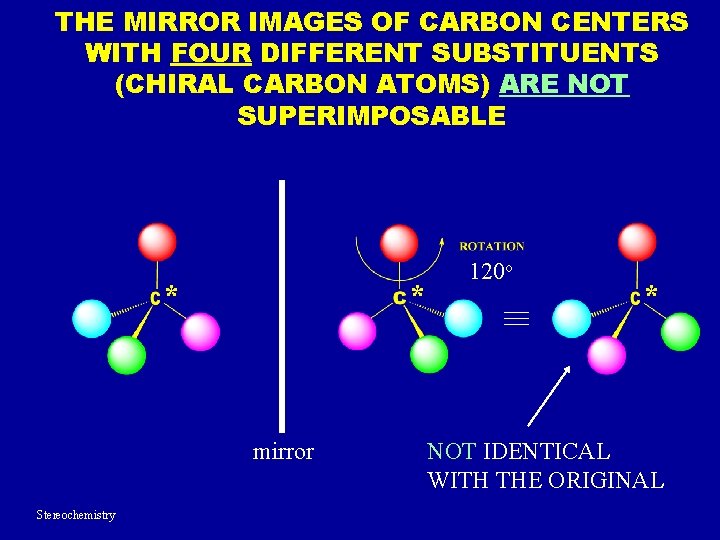
THE MIRROR IMAGES OF CARBON CENTERS WITH FOUR DIFFERENT SUBSTITUENTS (CHIRAL CARBON ATOMS) ARE NOT SUPERIMPOSABLE * * mirror Stereochemistry 120 o * NOT IDENTICAL WITH THE ORIGINAL

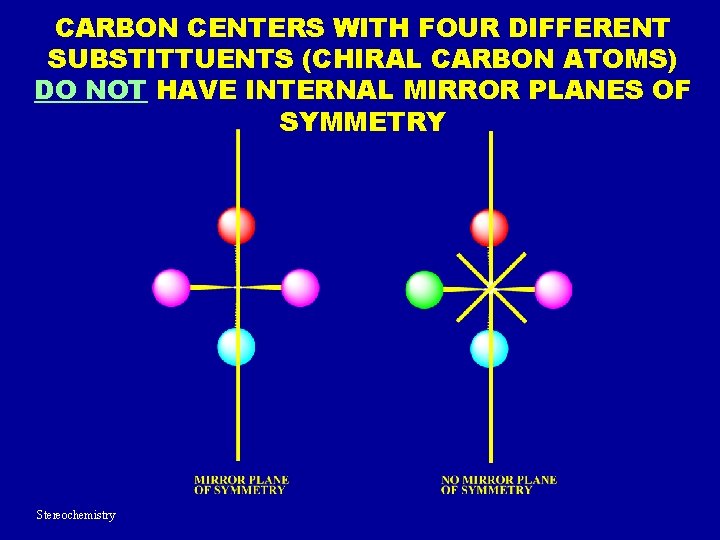
CARBON CENTERS WITH FOUR DIFFERENT SUBSTITTUENTS (CHIRAL CARBON ATOMS) DO NOT HAVE INTERNAL MIRROR PLANES OF SYMMETRY Stereochemistry

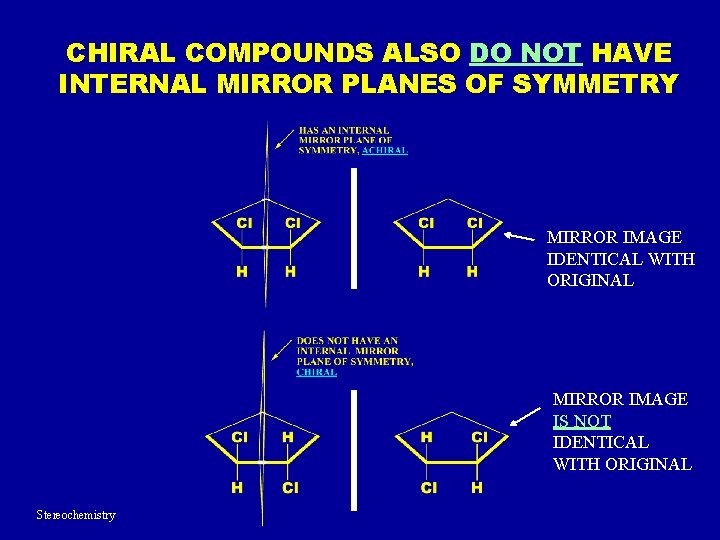
CHIRAL COMPOUNDS ALSO DO NOT HAVE INTERNAL MIRROR PLANES OF SYMMETRY MIRROR IMAGE IDENTICAL WITH ORIGINAL MIRROR IMAGE IS NOT IDENTICAL WITH ORIGINAL Stereochemistry

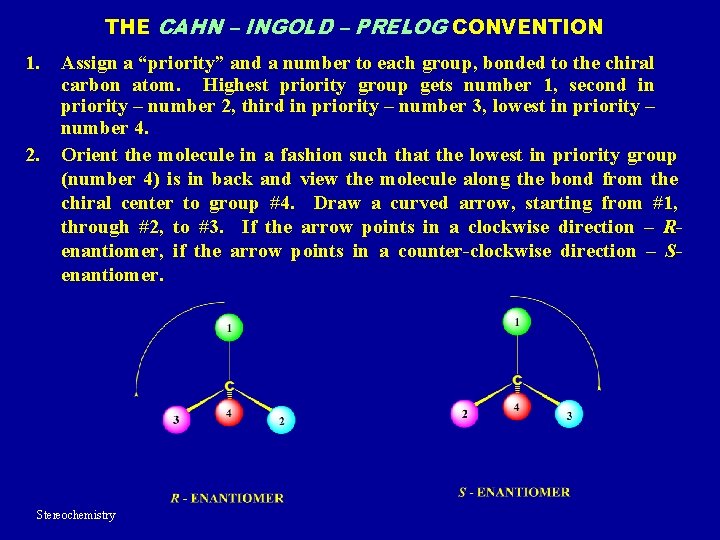
THE CAHN – INGOLD – PRELOG CONVENTION 1. 2. Assign a “priority” and a number to each group, bonded to the chiral carbon atom. Highest priority group gets number 1, second in priority – number 2, third in priority – number 3, lowest in priority – number 4. Orient the molecule in a fashion such that the lowest in priority group (number 4) is in back and view the molecule along the bond from the chiral center to group #4. Draw a curved arrow, starting from #1, through #2, to #3. If the arrow points in a clockwise direction – Renantiomer, if the arrow points in a counter-clockwise direction – Senantiomer. Stereochemistry

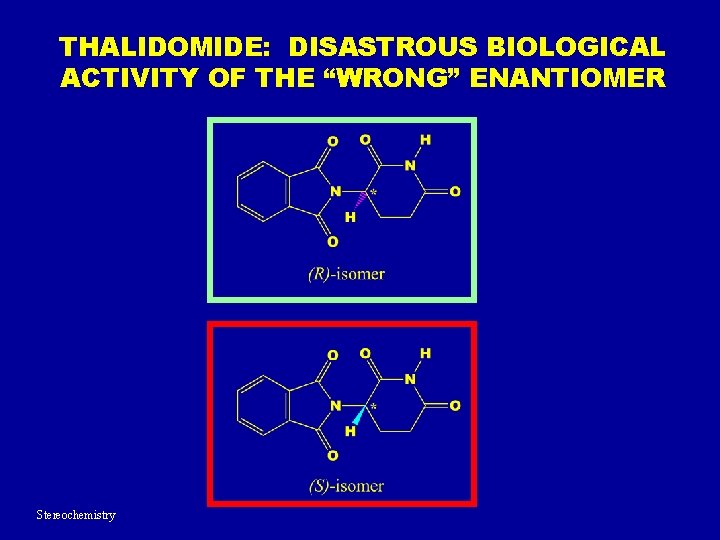
THALIDOMIDE: DISASTROUS BIOLOGICAL ACTIVITY OF THE “WRONG” ENANTIOMER Stereochemistry