Chapter 7 Stereochemistry 7 1 Molecular Chirality Enantiomers







- Slides: 30

Chapter 7 Stereochemistry

7. 1 Molecular Chirality: Enantiomers

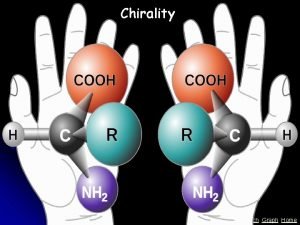
Chirality A molecule is chiral if its two mirror image forms are not superposable upon one another. A molecule is achiral if its two mirror image forms are superposable.

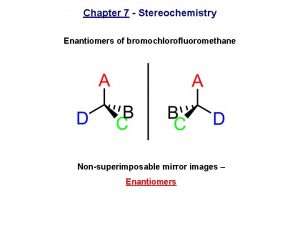
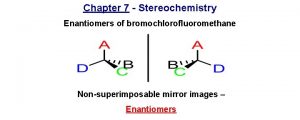
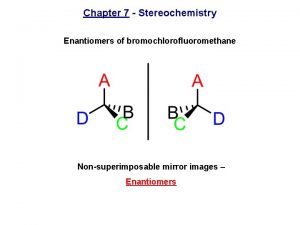
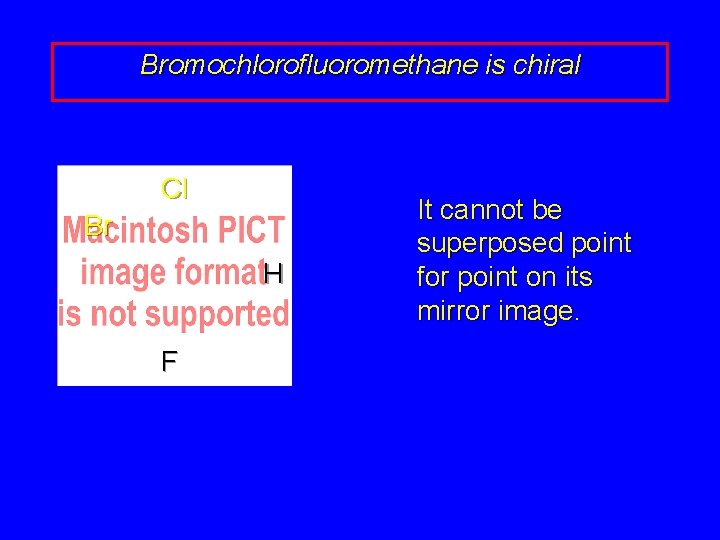
Bromochlorofluoromethane is chiral Cl Br H F It cannot be superposed point for point on its mirror image.

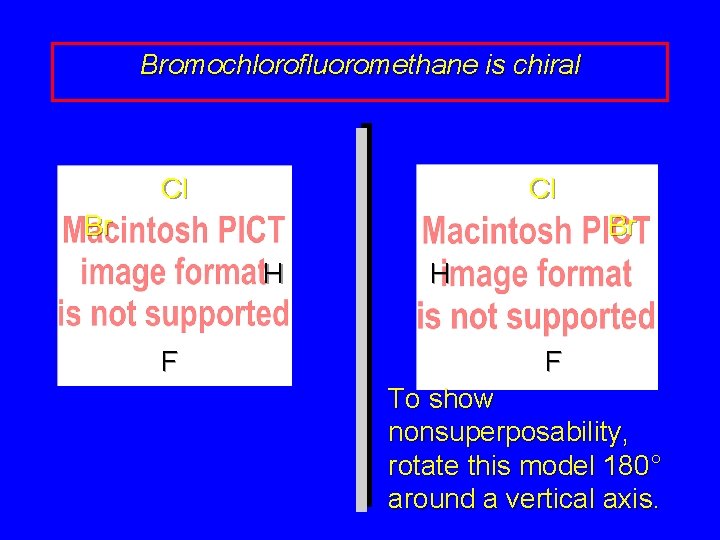
Bromochlorofluoromethane is chiral Cl Cl Br Br H F To show nonsuperposability, rotate this model 180° around a vertical axis.

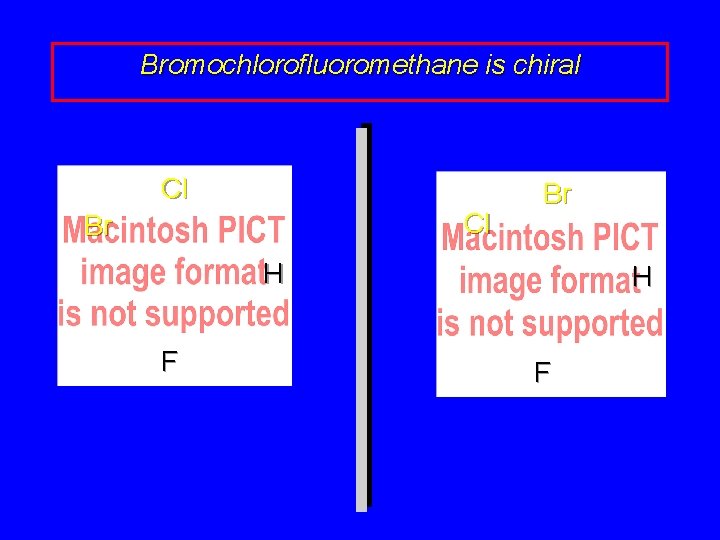
Bromochlorofluoromethane is chiral Cl Cl Br Br H F

Another look

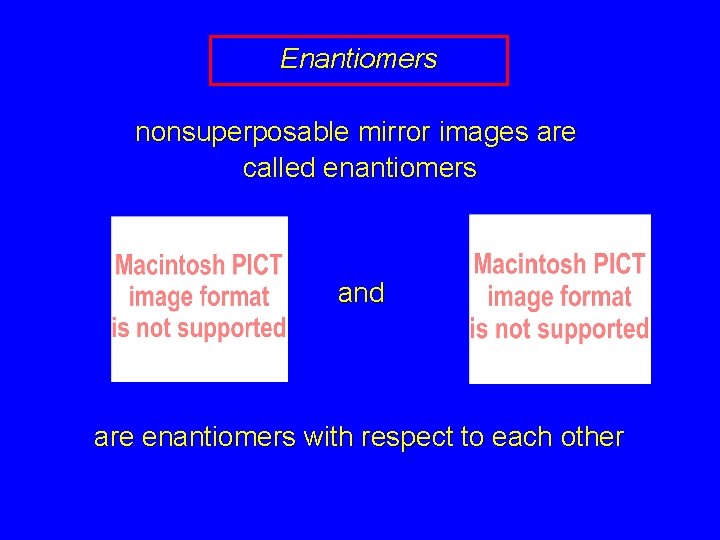
Enantiomers nonsuperposable mirror images are called enantiomers and are enantiomers with respect to each other

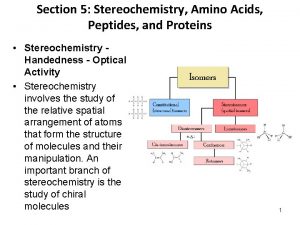
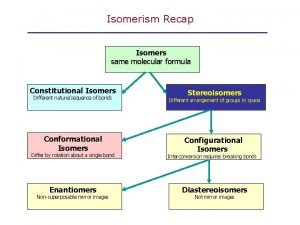
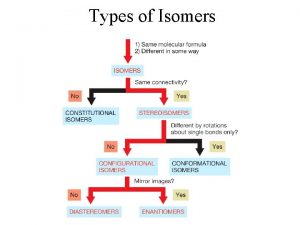
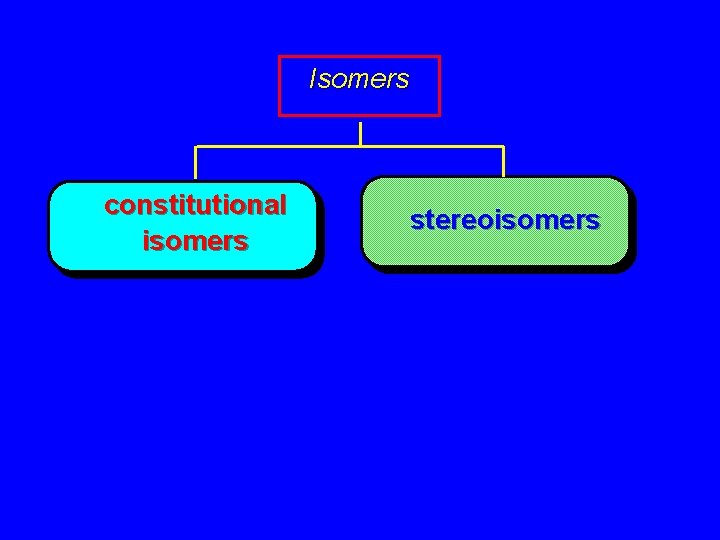
Isomers constitutional isomers stereoisomers

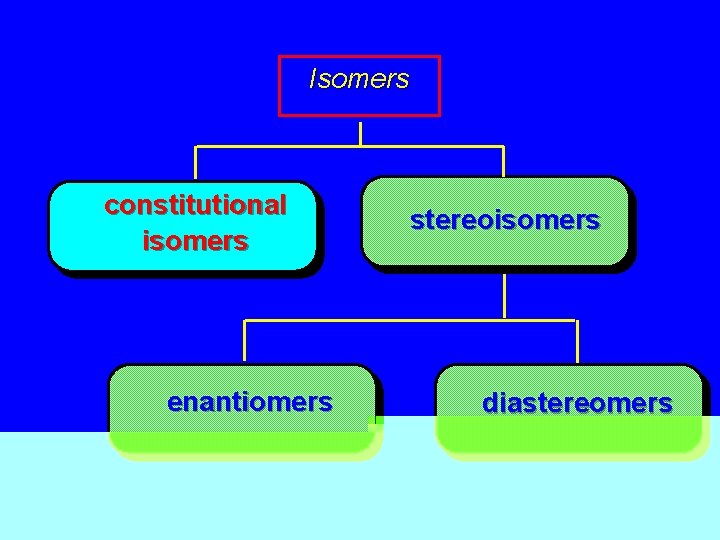
Isomers constitutional isomers enantiomers stereoisomers diastereomers

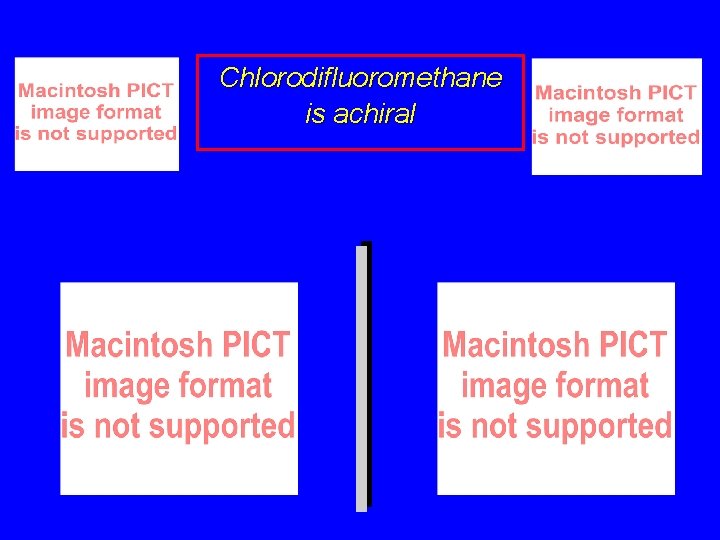
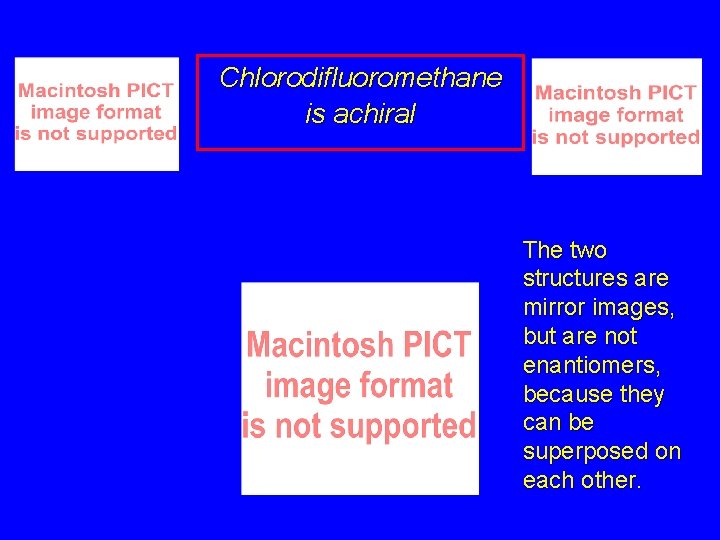
Chlorodifluoromethane is achiral

Chlorodifluoromethane is achiral The two structures are mirror images, but are not enantiomers, because they can be superposed on each other.

7. 2 The Stereogenic Center

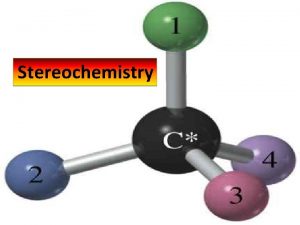
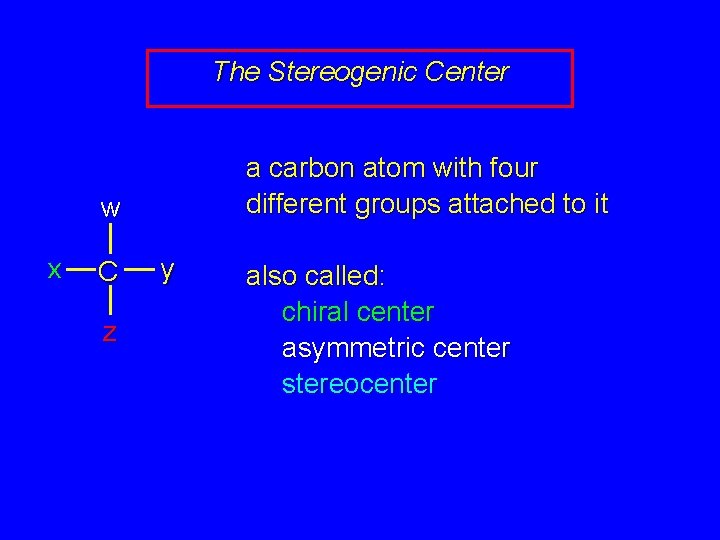
The Stereogenic Center a carbon atom with four different groups attached to it w x C z y also called: chiral center asymmetric center stereocenter

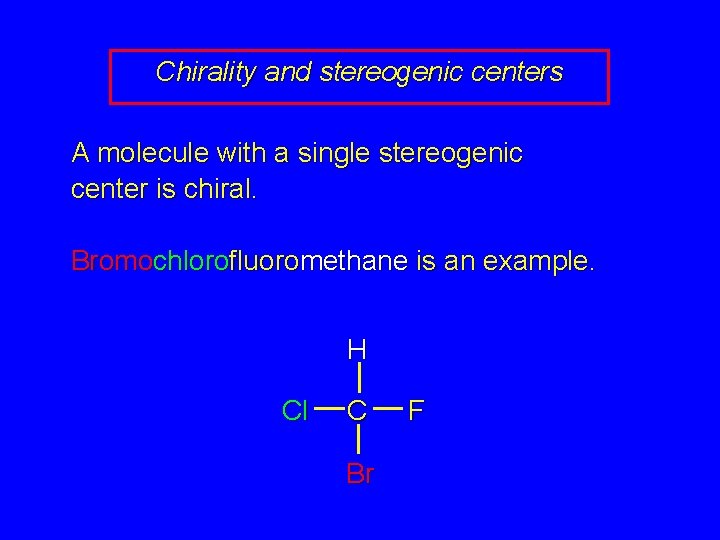
Chirality and stereogenic centers A molecule with a single stereogenic center is chiral. Bromochlorofluoromethane is an example. H Cl C Br F

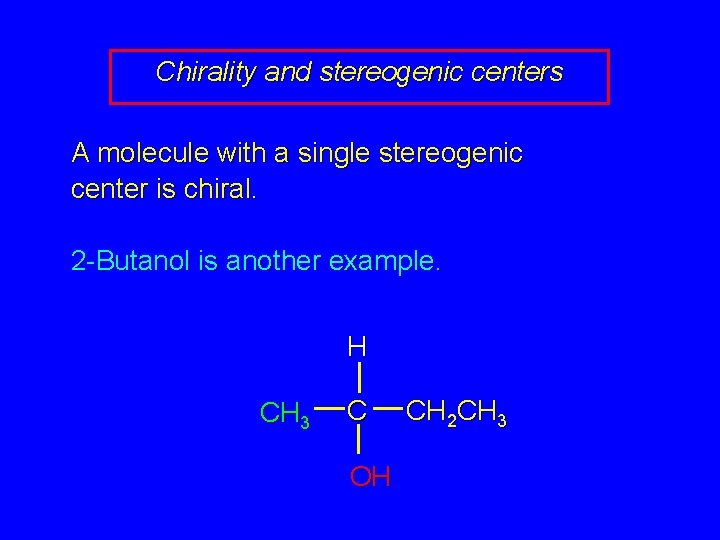
Chirality and stereogenic centers A molecule with a single stereogenic center is chiral. 2 -Butanol is another example. H CH 3 C OH CH 2 CH 3

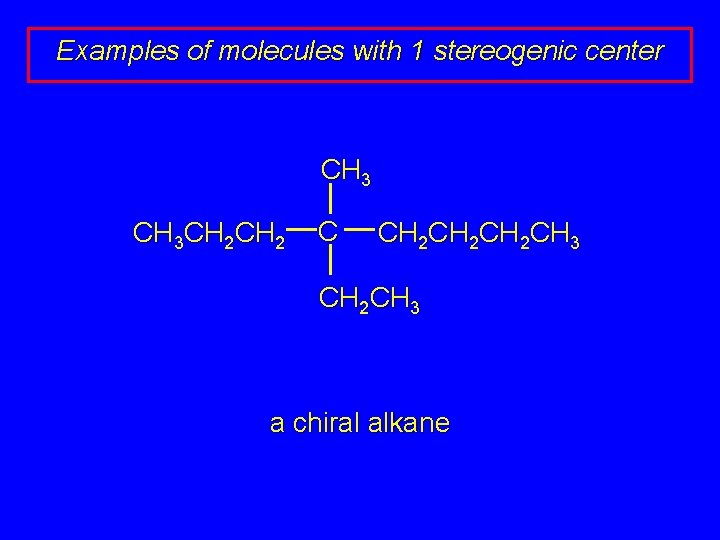
Examples of molecules with 1 stereogenic center CH 3 CH 2 C CH 2 CH 2 CH 3 a chiral alkane

Examples of molecules with 1 stereogenic center OH Linalool, a naturally occurring chiral alcohol

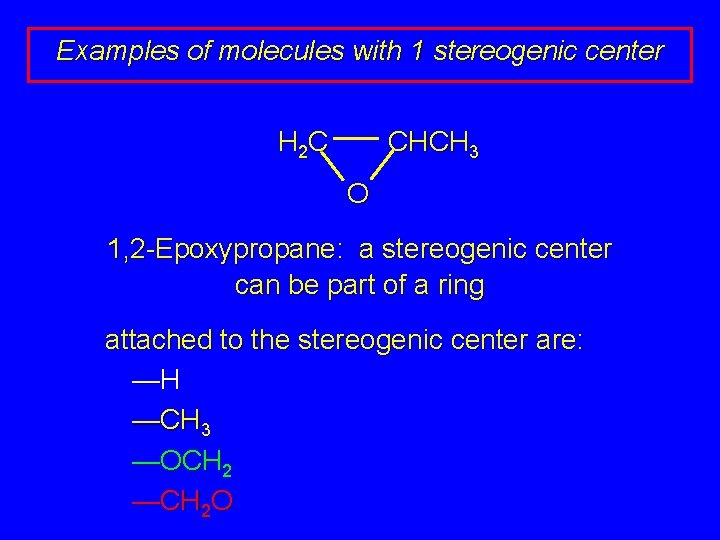
Examples of molecules with 1 stereogenic center H 2 C CHCH 3 O 1, 2 -Epoxypropane: a stereogenic center can be part of a ring attached to the stereogenic center are: —H —CH 3 —OCH 2 —CH 2 O

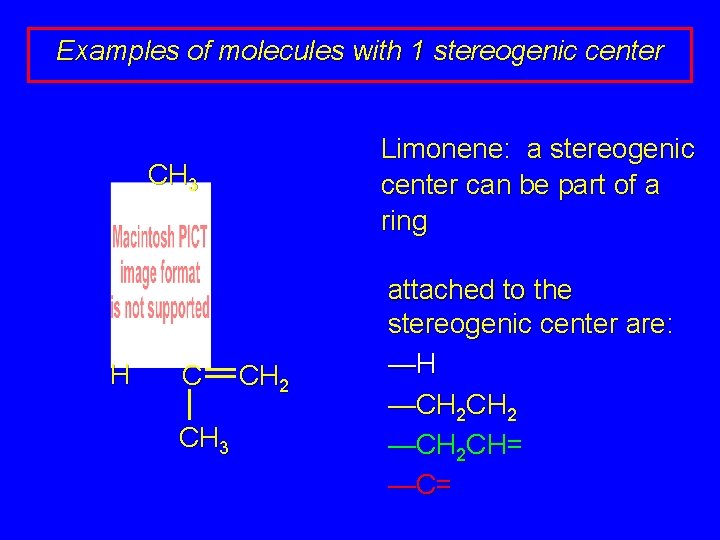
Examples of molecules with 1 stereogenic center Limonene: a stereogenic center can be part of a ring CH 3 H C CH 3 CH 2 attached to the stereogenic center are: —H —CH 2 CH= —C=

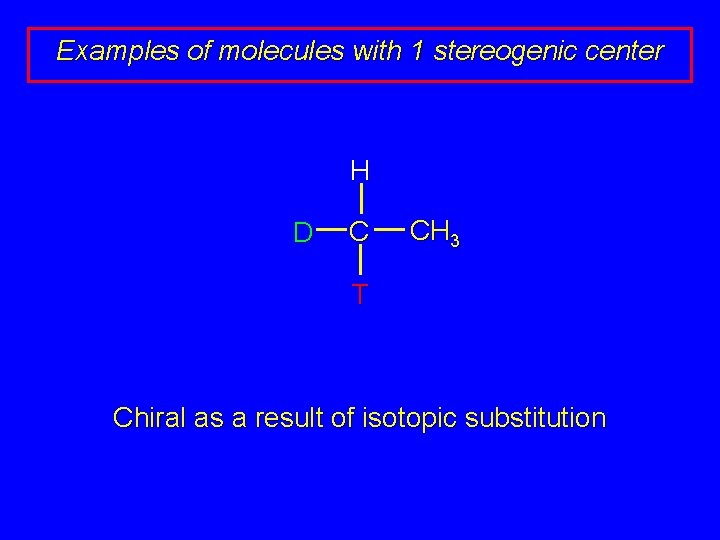
Examples of molecules with 1 stereogenic center H D C CH 3 T Chiral as a result of isotopic substitution

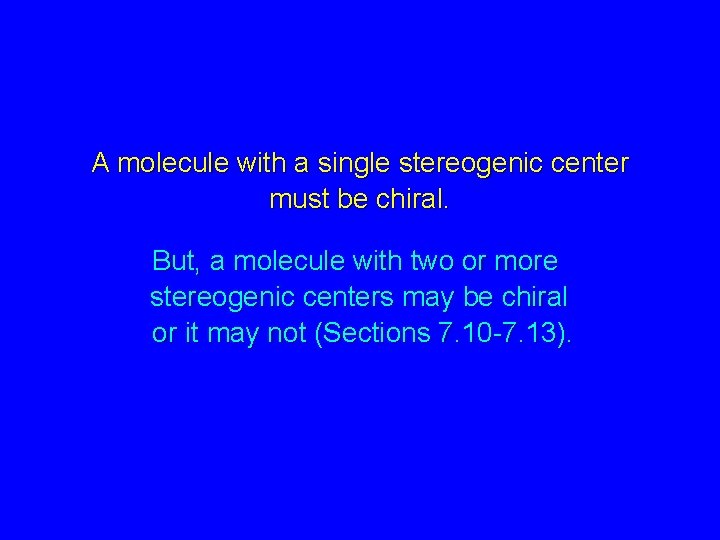
A molecule with a single stereogenic center must be chiral. But, a molecule with two or more stereogenic centers may be chiral or it may not (Sections 7. 10 -7. 13).

7. 3 Symmetry in Achiral Structures

Symmetry tests for achiral structures Any molecule with a plane of symmetry or a center of symmetry must be achiral.

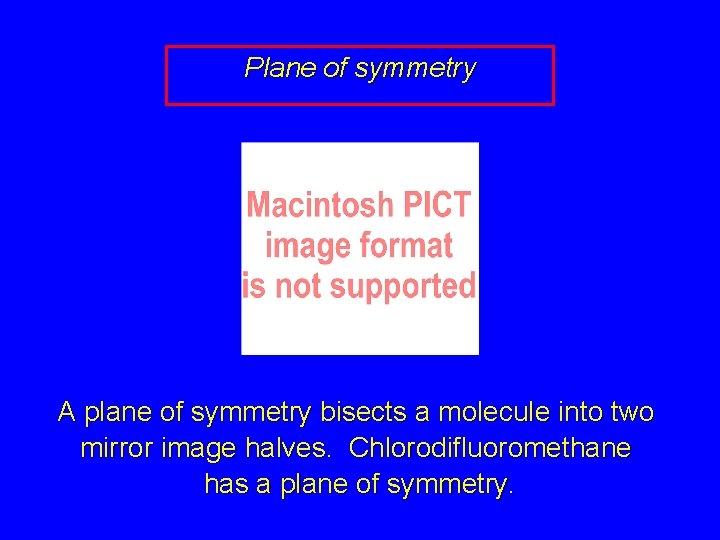
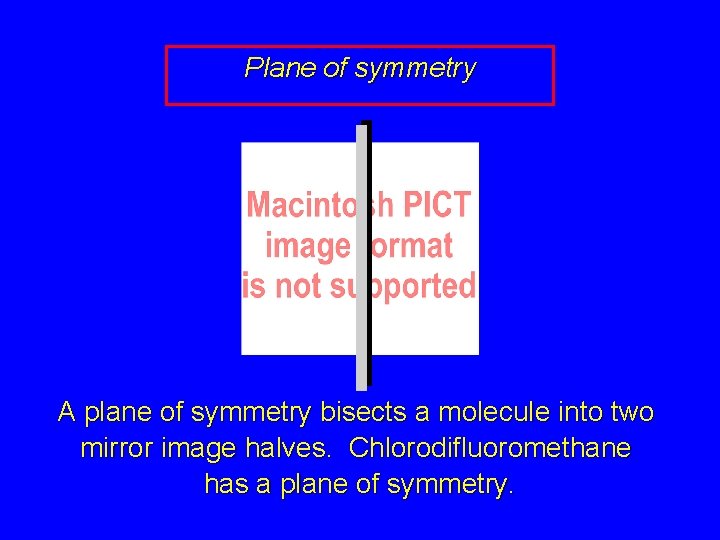
Plane of symmetry A plane of symmetry bisects a molecule into two mirror image halves. Chlorodifluoromethane has a plane of symmetry.

Plane of symmetry A plane of symmetry bisects a molecule into two mirror image halves. Chlorodifluoromethane has a plane of symmetry.

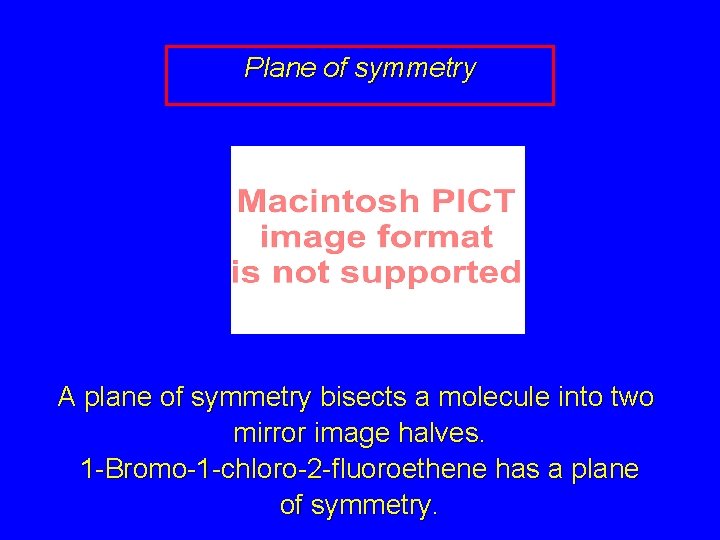
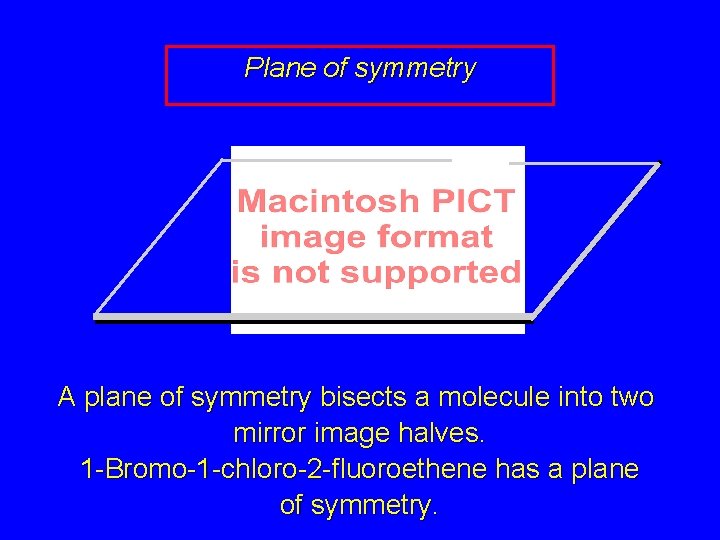
Plane of symmetry A plane of symmetry bisects a molecule into two mirror image halves. 1 -Bromo-1 -chloro-2 -fluoroethene has a plane of symmetry.

Plane of symmetry A plane of symmetry bisects a molecule into two mirror image halves. 1 -Bromo-1 -chloro-2 -fluoroethene has a plane of symmetry.

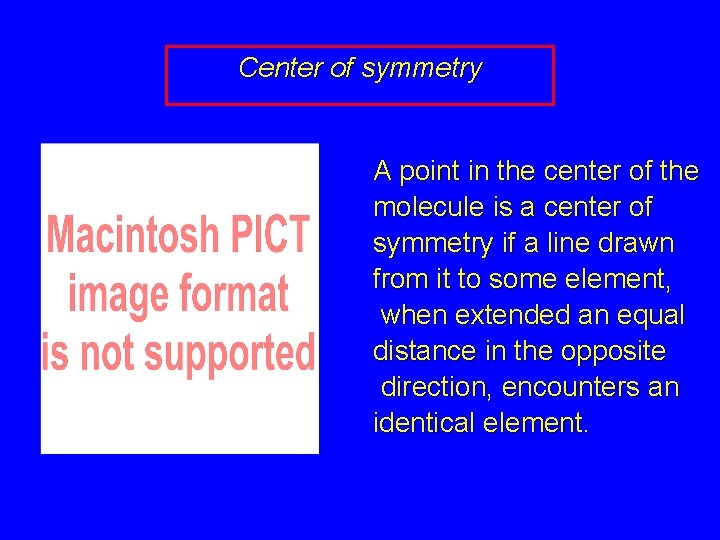
Center of symmetry A point in the center of the molecule is a center of symmetry if a line drawn from it to some element, when extended an equal distance in the opposite direction, encounters an identical element.

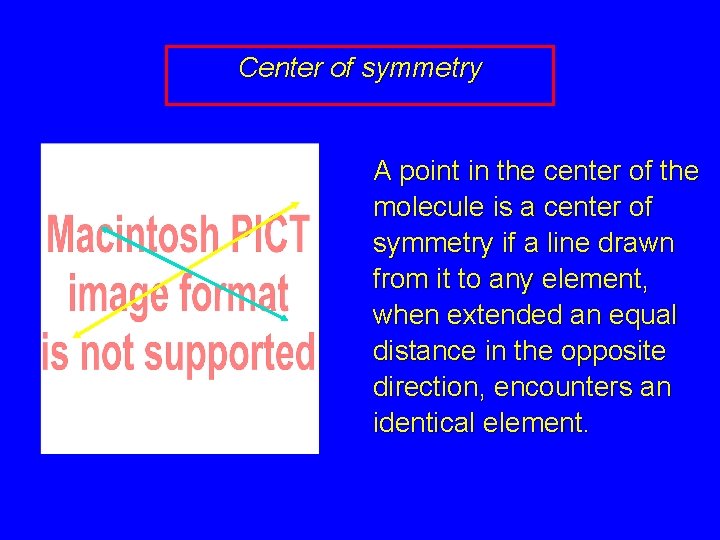
Center of symmetry A point in the center of the molecule is a center of symmetry if a line drawn from it to any element, when extended an equal distance in the opposite direction, encounters an identical element.
 Planar chirality
Planar chirality Virginie simonet
Virginie simonet Carbon carbon bonds
Carbon carbon bonds Chirality
Chirality Chirality centers in sucralose
Chirality centers in sucralose Early man
Early man Example of denatured protein
Example of denatured protein 2 3 4-trihydroxybutanal stereoisomers
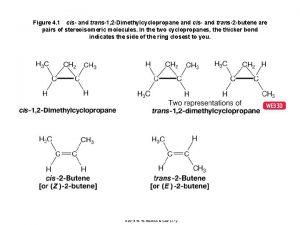
2 3 4-trihydroxybutanal stereoisomers Cyclopentane cis trans isomers
Cyclopentane cis trans isomers Indicate the relationship of the pair of molecules shown.
Indicate the relationship of the pair of molecules shown. Father of stereochemistry
Father of stereochemistry Chiral vs achiral
Chiral vs achiral Stereochemistry
Stereochemistry Ethane
Ethane Stereochemistry of cephalosporin
Stereochemistry of cephalosporin Introduction to stereochemistry
Introduction to stereochemistry Stereoisomer vs constitutional isomer
Stereoisomer vs constitutional isomer Cyclisation of fructose
Cyclisation of fructose Bromochlorofluoromethane enantiomers
Bromochlorofluoromethane enantiomers Enantiomers vs epimers
Enantiomers vs epimers Properties of diastereomers
Properties of diastereomers Resolution of enantiomers
Resolution of enantiomers Nonsuperposable
Nonsuperposable Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Chapter 16 the molecular basis of inheritance
Chapter 16 the molecular basis of inheritance Chapter 12 section 1 molecular genetics answer key
Chapter 12 section 1 molecular genetics answer key Chapter 12 molecular genetics answer key
Chapter 12 molecular genetics answer key Molecular biology of the gene chapter 10
Molecular biology of the gene chapter 10 Chapter 16 the molecular basis of inheritance
Chapter 16 the molecular basis of inheritance