PROPERTIES OF ENANTIOMERS IN FOOD DLVN ZKAN Enantiomers













- Slides: 25

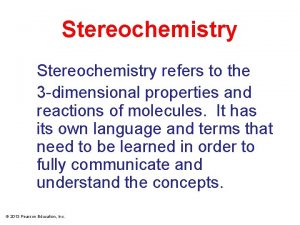
PROPERTIES OF ENANTIOMERS IN FOOD DİLVİN ÖZKAN

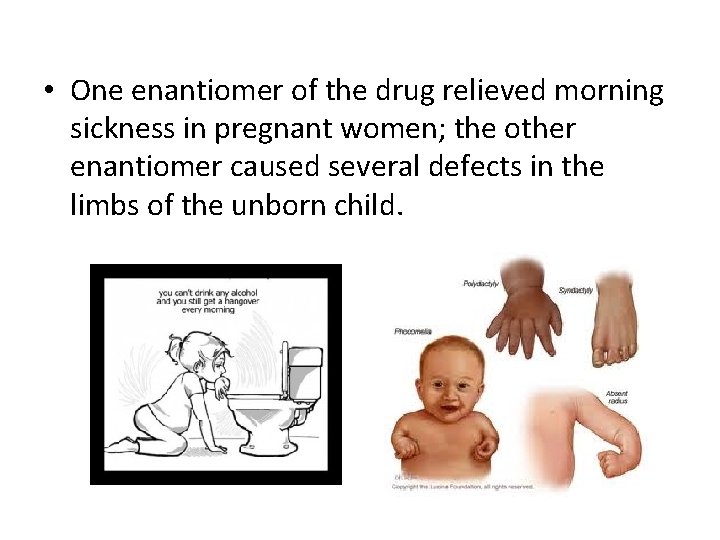
• Enantiomers can clearly have different biological effects. The importance of this with drugs was tragically shown with the drug thalidomide in the last century. 1962 photo of a baby born to a mother who had taken thalidomide while pregnant; the baby has an extra appendage connected to the foot and the malformation of the right arm

• One enantiomer of the drug relieved morning sickness in pregnant women; the other enantiomer caused several defects in the limbs of the unborn child.

• Foods, too, can have different effects. The Dform of vitamin C, for example, has no biological activity. • Different enantiomeric forms may vary in their tastes, odour and toxicity.

• Most naturally occuring sugars exist in the Dform, whereas most naturally occurring amino acids are in the L- form. • D- amino acids tend to taste sweet, whereas Lforms often have no taste.

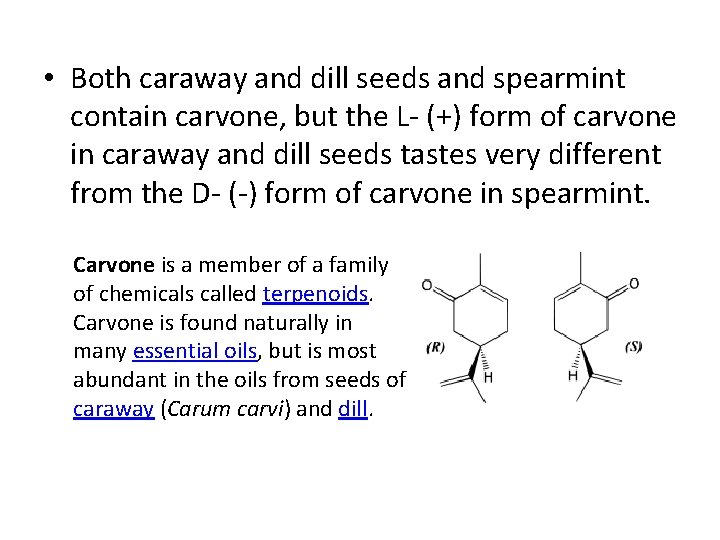
• Both caraway and dill seeds and spearmint contain carvone, but the L- (+) form of carvone in caraway and dill seeds tastes very different from the D- (-) form of carvone in spearmint. Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway (Carum carvi) and dill.


• Smells and tastes can appear different people, because the olfactory receptors also contain chiral receptor molecules, which can interact differently with the enantiomeric molecules in food.

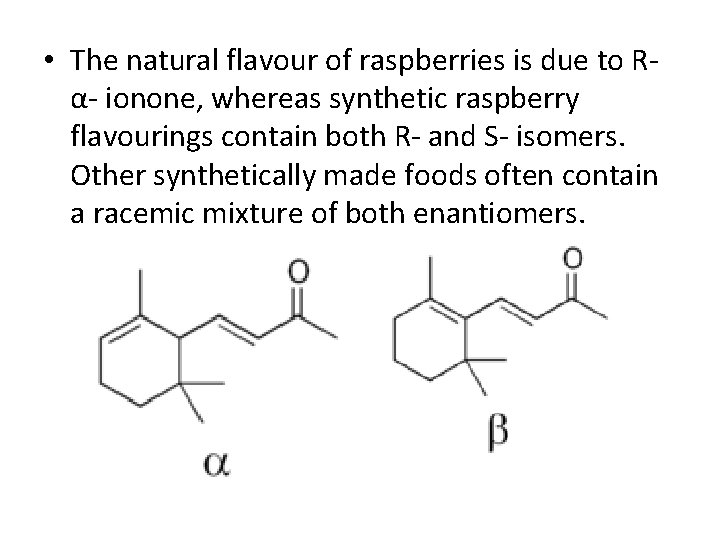
• The natural flavour of raspberries is due to Rα- ionone, whereas synthetic raspberry flavourings contain both R- and S- isomers. Other synthetically made foods often contain a racemic mixture of both enantiomers.

• The ionones are a series of closely related chemical substances that are part of a group of compounds known as rose ketones. Ionones are aroma compounds found in a variety of essential oils, including rose oil. beta-Ionone is a significant contributor to the aroma of roses, despite its relatively low concentration, and is an important fragrance chemical used in perfumery. The ionones are derived from the degradation of carotenoids.

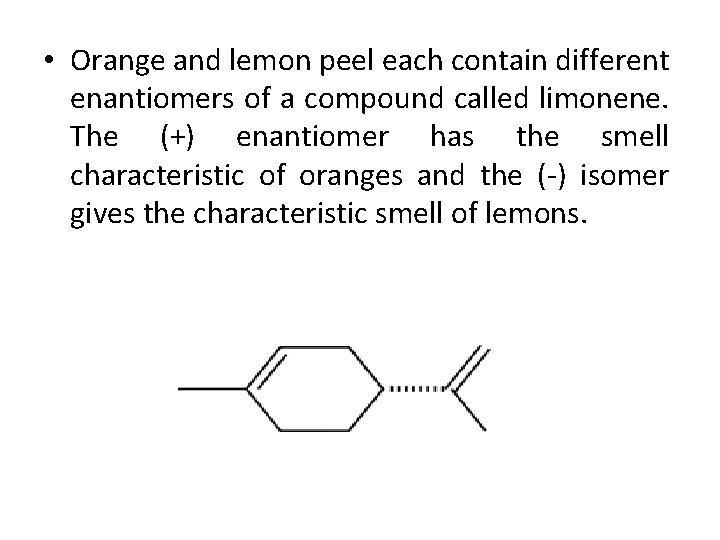
• Orange and lemon peel each contain different enantiomers of a compound called limonene. The (+) enantiomer has the smell characteristic of oranges and the (-) isomer gives the characteristic smell of lemons.

• Limonene is a colourless liquid hydrocarbon classified as a cyclic terpene possessing a strong smell of oranges. It is used in chemical synthesis as a precursor to carvone and as a renewably-based solvent in cleaning products.


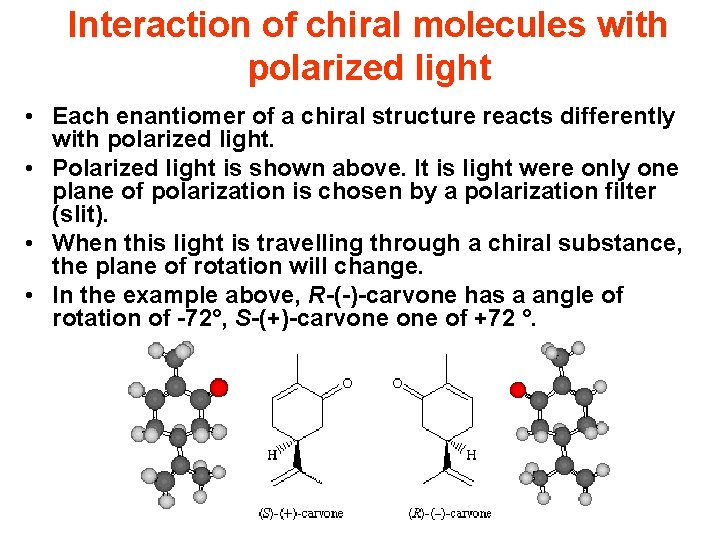
Interaction of chiral molecules with polarized light • Each enantiomer of a chiral structure reacts differently with polarized light. • Polarized light is shown above. It is light were only one plane of polarization is chosen by a polarization filter (slit). • When this light is travelling through a chiral substance, the plane of rotation will change. • In the example above, R-(-)-carvone has a angle of rotation of -72°, S-(+)-carvone of +72 °.

A polaroid filter allows light through only if the light is polarized at the same angle as the filter.

Why Polarized Light Is Affected • So why do chiral molecules affect only polarized light, and not unpolarized? • Well, they do affect unpolarized light, but since the rays have no particular orientation to one another, the effect can not be observed or measured. • We observe the polarized light rays being rotated because we knew their orientation before passing through the chiral substance, and so we can measure the degree of change afterwards. • What happens is this; when light passes through matter, e. g. a solution containing either chiral or achiral molecules, the light is actually interacting with each molecule's electron cloud, and these very interactions can result in the rotation of the plane of oscillation for a ray of light.

Why Polarized Light Is Affected • The direction and magnitude of rotation depends on the nature of the electron cloud, so it stands to reason that two identical molecules possessing identical electron clouds will rotate light in the exact same manner. • This is why achiral molecules do not exhibit optical activity. • In a chiral solution that is not a racemic mixture, however, the chiral molecules present in greater numbers are configurationally equivalent to each other, and therefore each possesses identical electron clouds to its molecular twins. • As such, each interaction between light and one of these 'majority' molecule's electron clouds will result in rotations of identical magnitude and direction.

Why Polarized Light Is Affected • When these billions of interactions are summed together into one cohesive number, they do not cancel one another as racemic and achiral solutions tend to do - rather, the chiral solution as a whole is observed to rotate polarized light in one particular direction due to its molecular properties.

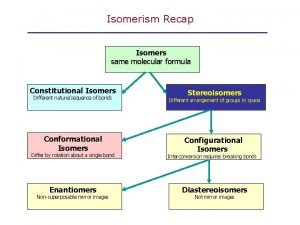
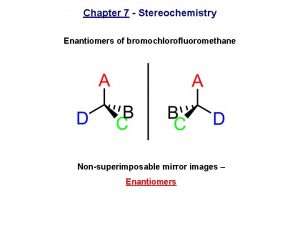
Enantiomers • It is just such specificity that accounts for the optical isomerism of enantiomeric compounds. • Enantiomers possess identical chemical structures (i. e. their atoms are the same and connected in the same order), but are mirror images of one another. • Therefore, their electron clouds are also identical but actually mirror images of one another and not superimposable. • For this reason, enantiomeric pairs rotate light by the same magnitude (number of degrees), but they each rotate plane polarized light in opposite directions. • If one chiral version has the property of rotating polarized light to the right (clockwise), it only makes sense that the molecule's chiral mirror image would rotate light to the left (counterclockwise). • Equal amounts of each enantiomer results in no rotation. Mixtures of this type are called racemic mixtures, and they behave much as achiral molecules do.

Difference between D and L • Chiral (Greek, cheir = hand) molecule is not superimposible on its mirror image while in other case achiral molecule is superimposible on its mirror image. • The chirality of the molecule in nearly all cases is appropriate to the presence of a single chiral (asymmetric atom). • A chiral atom is any tetrahedral atom with four unlike groups attached to it and is also known as chiral centre. • Enantiomorphs exist only in case of chiral molecules. • The terms chiral and achiral are also used to designate dissymmetric and non-disymmetric molecules respectively.

Optical Activity of D and L • The majority of the physical and chemical properties of the optical isomers are equal. • Since the occurrence of optical isomerism is caused by the asymmetry of the molecules the discovery of the difference in the properties can be measured only when an asymmetric measuring instrument is used. • The most common of such tool is planepolarized light. • Plane polarized light is defined as the light, whose vibrations happen in a single plane only is known as plane polarized light and the phenomenon is known as polarization.

Optical Activity of D and L • When plane polarized light is passed throughout certain substances or their solutions its plane of polarization is rotated moreover towards right (clockwise) or towards left (anti-clockwise) by a certain angle. • The substances which rotate or turn the plane of polarization of the plane polarized light are known as optically active and the phenomenon is referred to as optical activity. • Those substances which rotate the plane polarized light to right are called dextro-rotatory indicated by the sign‘d’ or (+) and those which rotate to the left are called levorotatory indicated by the sign ‘l’ or (-).

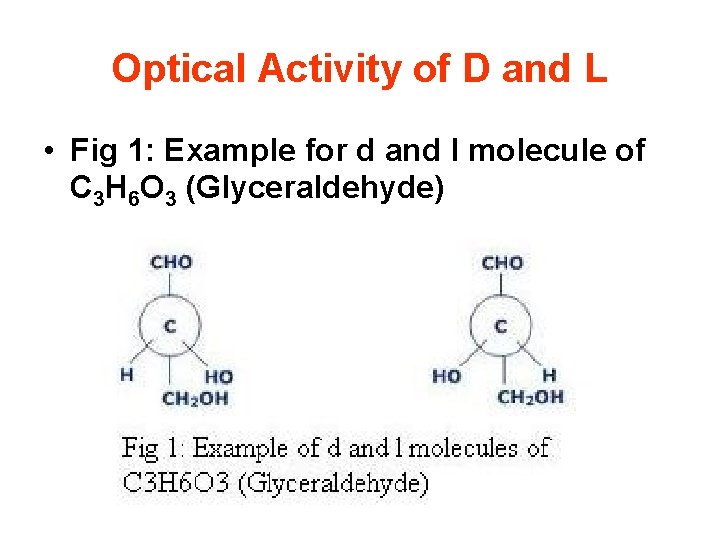
Optical Activity of D and L • Fig 1: Example for d and l molecule of C 3 H 6 O 3 (Glyceraldehyde)

Difference between D and L The extent of rotation depends on the following factors: • Nature of the substance. • Wavelength of the light used. • Concentration of the solution (if the substance is taken in solution). • Thickness of the layer or length of path through which polarized light passes. • Nature of the solvent (for solutions only). • Temperature of measurements. • The apparatus used to measure the optical activity is known as polarimeter and has been schematically represented by the diagram. • The optical inactivity could also arise due to the presence of equal amounts of the dextro and levo forms when the compound is prepared or obtained by mixing the d and l forms in equal molecular proportions.

REFERENCES • http: //en. wikibooks. org/wiki/Organic_Chem istry/Chirality/Optical_activity
 Gencon 76
Gencon 76 Ie
Ie Diastereomers examples
Diastereomers examples Cyclisation of fructose
Cyclisation of fructose Nonsuperimposable
Nonsuperimposable R vs s configuration
R vs s configuration Enantiomers vs epimers
Enantiomers vs epimers Are enantiomers optically active
Are enantiomers optically active Resolution of enantiomers
Resolution of enantiomers Nonsuperimposible
Nonsuperimposible Unit 2 food food food
Unit 2 food food food Food chain sequence
Food chain sequence Extensive properties and intensive properties
Extensive properties and intensive properties Chemical properties of citric acid
Chemical properties of citric acid Disupear
Disupear Properties of food
Properties of food Food scientists measure food energy in:
Food scientists measure food energy in: Inspects retail and foodservice operations
Inspects retail and foodservice operations Tcs food
Tcs food Food quantifiers exercise
Food quantifiers exercise Third trophic level
Third trophic level Food handlers can contaminate food when they
Food handlers can contaminate food when they Food product design from fast food nation by eric schlosser
Food product design from fast food nation by eric schlosser Sample food chains
Sample food chains Food chains with 6 organisms
Food chains with 6 organisms How does the food chain go
How does the food chain go