MondayTuesday Thermodynamics of aqueous solutions Ion association Pitzer





![Mass-Action Equations Ca+2 + SO 4 -2 = Ca. SO 40 [] indicates activity Mass-Action Equations Ca+2 + SO 4 -2 = Ca. SO 40 [] indicates activity](https://slidetodoc.com/presentation_image/4d6b98f8dda8f663eadd138445bc6fb3/image-15.jpg)


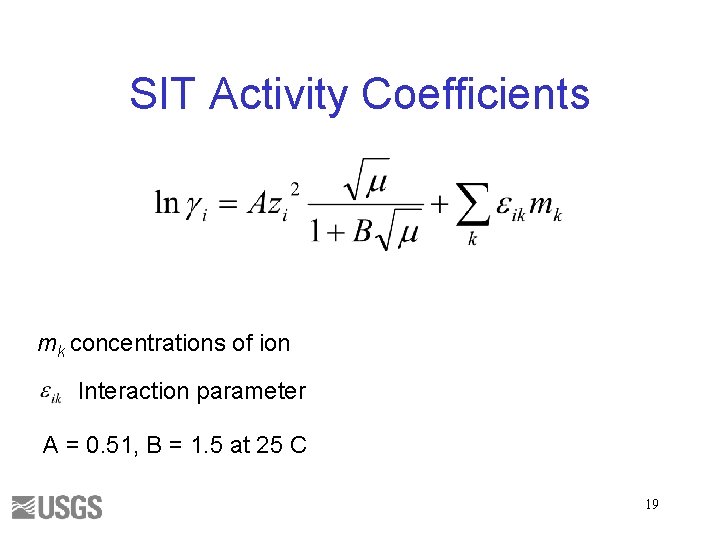






![What is p. H? p. H = 6. 3 + log[(HCO 3 -)/(CO 2)] What is p. H? p. H = 6. 3 + log[(HCO 3 -)/(CO 2)]](https://slidetodoc.com/presentation_image/4d6b98f8dda8f663eadd138445bc6fb3/image-52.jpg)
![What is pe? Fe+2 = Fe+3 + epe = log( [Fe+3]/[Fe+2] ) + 13 What is pe? Fe+2 = Fe+3 + epe = log( [Fe+3]/[Fe+2] ) + 13](https://slidetodoc.com/presentation_image/4d6b98f8dda8f663eadd138445bc6fb3/image-57.jpg)












- Slides: 88

Monday-Tuesday • Thermodynamics of aqueous solutions – Ion association – Pitzer – SIT • SOLUTION – – – Units p. H—ratio of HCO 3 -/CO 2 pe—ratio of oxidized/reduced valence states Charge balance Phase boundaries • Saturation indices – Uncertainties – Useful minerals • Identify potential reactants 1

PHREEQC Programs • PHREEQC Version 3 – PHREEQC: Batch with Charting – Phreeqc. I: GUI with Charting – IPhreeqc: Module for programming and scripting • PHAST – – Serial—soon to be Multithreaded Parallel—MPI for transport and chemistry TVD (not done) 4 Windows—GUI just accepted • WEBMOD-Watershed reactive transport 2

Solutions 3

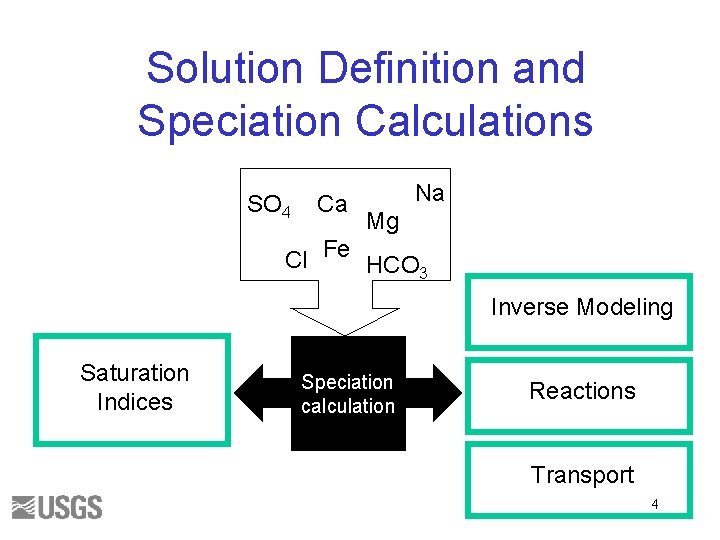
Solution Definition and Speciation Calculations SO 4 Ca Na Mg Cl Fe HCO 3 Inverse Modeling Saturation Indices Speciation calculation Reactions Transport 4

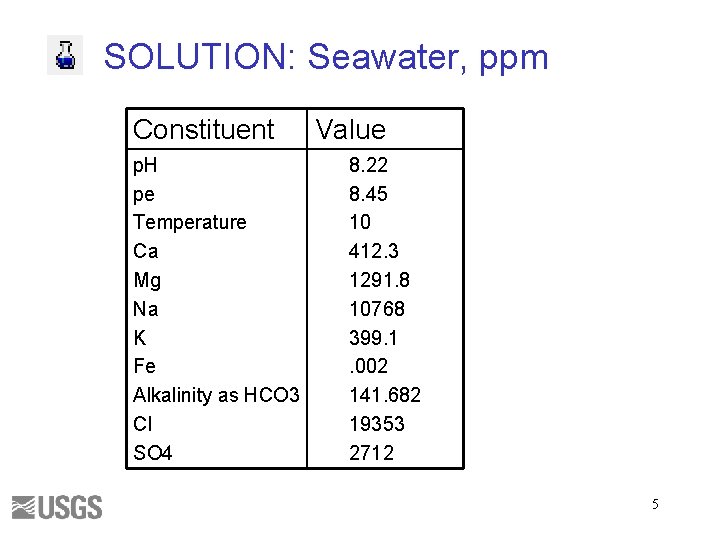
SOLUTION: Seawater, ppm Constituent p. H pe Temperature Ca Mg Na K Fe Alkalinity as HCO 3 Cl SO 4 Value 8. 22 8. 45 10 412. 3 1291. 8 10768 399. 1. 002 141. 682 19353 2712 5

Periodic_table. bmp 6

Initial Solution 1. Questions 1. What is the approximate molality of Ca? 2. What is the approximate alkalinity in meq/kgw? 3. What is the alkalinity concentration in mg/kgs as Ca. CO 3? 4. What effect does density have on the calculated molality? PHREEQC results are always moles or molality 7

Initial Solution 1. For most waters, we can assume most of the mass in solution is water. Mass of water in 1 kg seawater ~ 1 kg. 1. 412/40 ~ 10 mmol/kgw ~ 0. 01 molal 2. 142/61 ~ 2. 3 meq/kgw ~ 0. 0023 molal 3. 2. 3*50 ~ 116 mg/kgw as Ca. CO 3 4. None, density will only be used when concentration is specified as per liter. 8

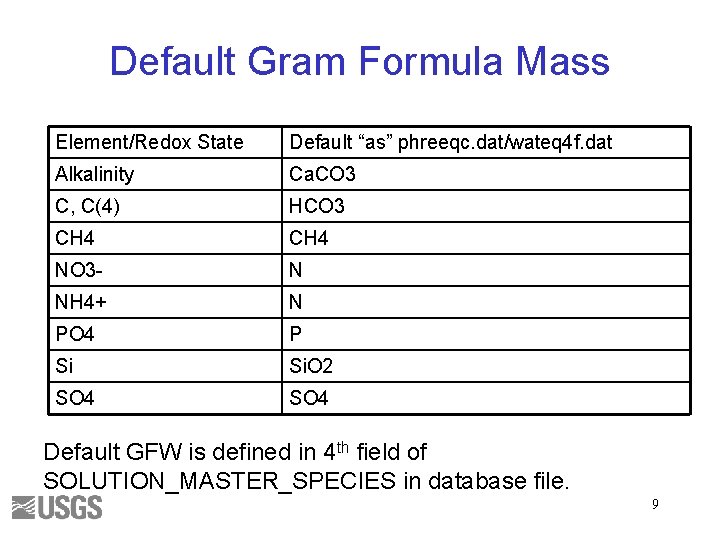
Default Gram Formula Mass Element/Redox State Default “as” phreeqc. dat/wateq 4 f. dat Alkalinity Ca. CO 3 C, C(4) HCO 3 CH 4 NO 3 - N NH 4+ N PO 4 P Si Si. O 2 SO 4 Default GFW is defined in 4 th field of SOLUTION_MASTER_SPECIES in database file. 9

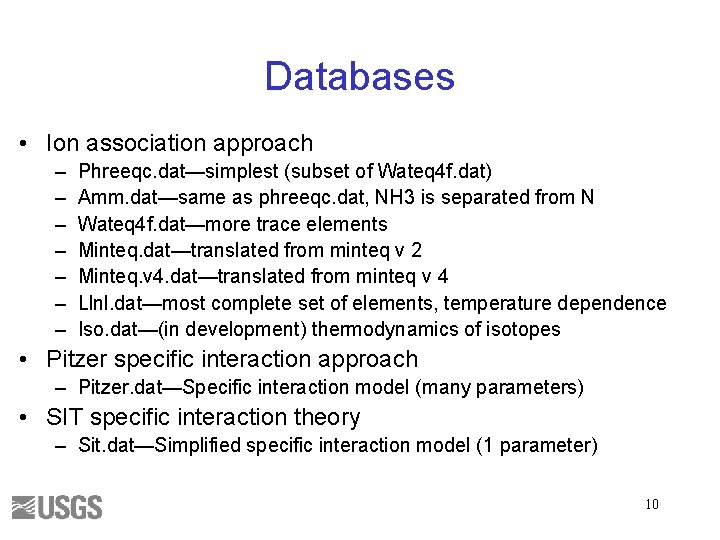
Databases • Ion association approach – – – – Phreeqc. dat—simplest (subset of Wateq 4 f. dat) Amm. dat—same as phreeqc. dat, NH 3 is separated from N Wateq 4 f. dat—more trace elements Minteq. dat—translated from minteq v 2 Minteq. v 4. dat—translated from minteq v 4 Llnl. dat—most complete set of elements, temperature dependence Iso. dat—(in development) thermodynamics of isotopes • Pitzer specific interaction approach – Pitzer. dat—Specific interaction model (many parameters) • SIT specific interaction theory – Sit. dat—Simplified specific interaction model (1 parameter) 10

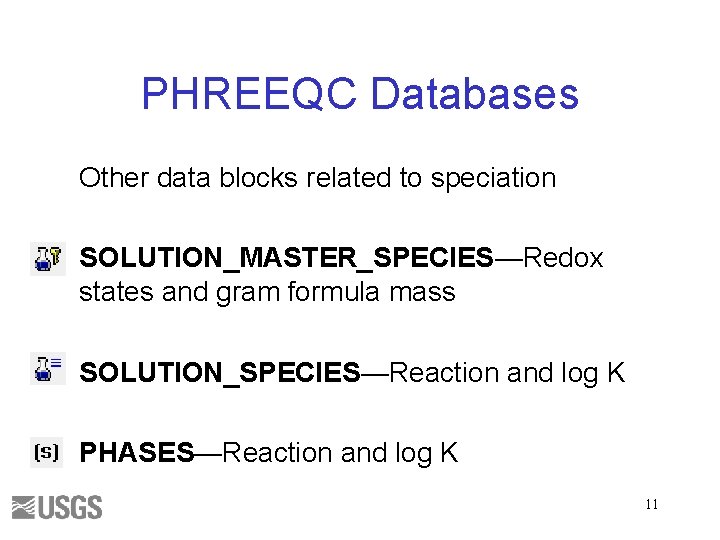
PHREEQC Databases Other data blocks related to speciation SOLUTION_MASTER_SPECIES—Redox states and gram formula mass SOLUTION_SPECIES—Reaction and log K PHASES—Reaction and log K 11

Solutions • Required for all PHREEQC calculations • SOLUTION and SOLUTION _SPREAD – – – Units p. H pe Charge balance Phase boundaries • Saturation indices – Useful minerals – Identify potential reactants 12

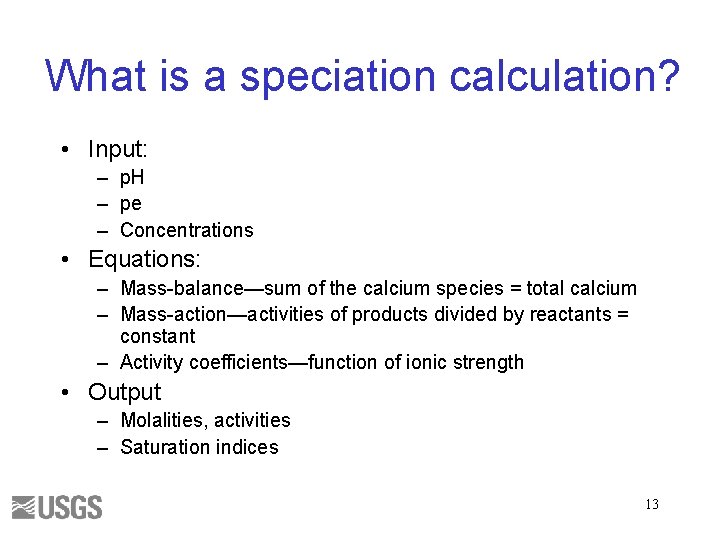
What is a speciation calculation? • Input: – p. H – pe – Concentrations • Equations: – Mass-balance—sum of the calcium species = total calcium – Mass-action—activities of products divided by reactants = constant – Activity coefficients—function of ionic strength • Output – Molalities, activities – Saturation indices 13

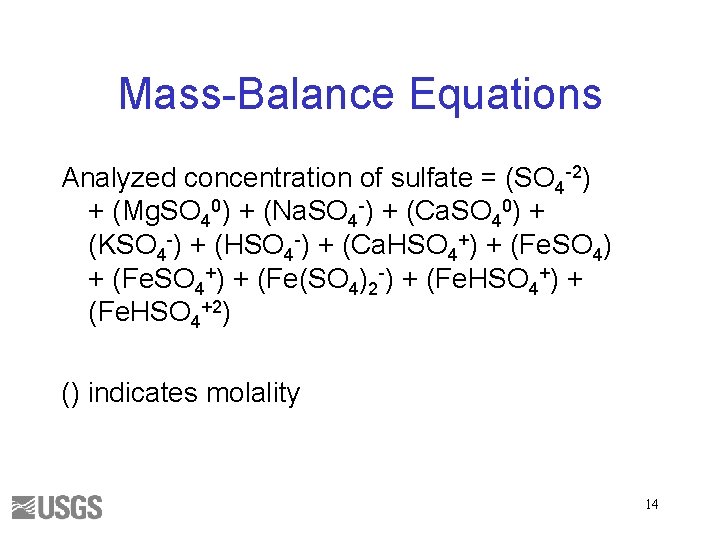
Mass-Balance Equations Analyzed concentration of sulfate = (SO 4 -2) + (Mg. SO 40) + (Na. SO 4 -) + (Ca. SO 40) + (KSO 4 -) + (HSO 4 -) + (Ca. HSO 4+) + (Fe(SO 4)2 -) + (Fe. HSO 4+2) () indicates molality 14
![MassAction Equations Ca2 SO 4 2 Ca SO 40 indicates activity Mass-Action Equations Ca+2 + SO 4 -2 = Ca. SO 40 [] indicates activity](https://slidetodoc.com/presentation_image/4d6b98f8dda8f663eadd138445bc6fb3/image-15.jpg)
Mass-Action Equations Ca+2 + SO 4 -2 = Ca. SO 40 [] indicates activity 15

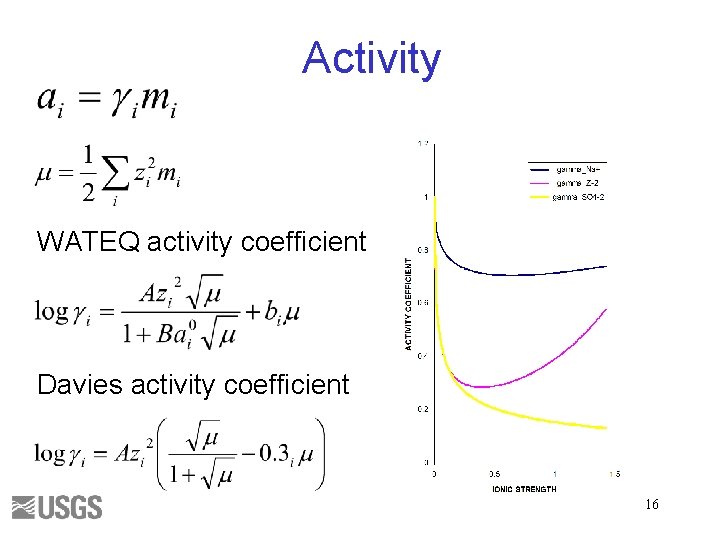
Activity WATEQ activity coefficient Davies activity coefficient 16

Uncharged Species bi, called the Setschenow coefficient Value of 0. 1 used in phreeqc. dat, wateq 4 f. dat. 17

Pitzer Activity Coefficients ma concentration of anion mc concentration of cation Ion specific parameters F function of ionic strength, molalities of cations and anions 18
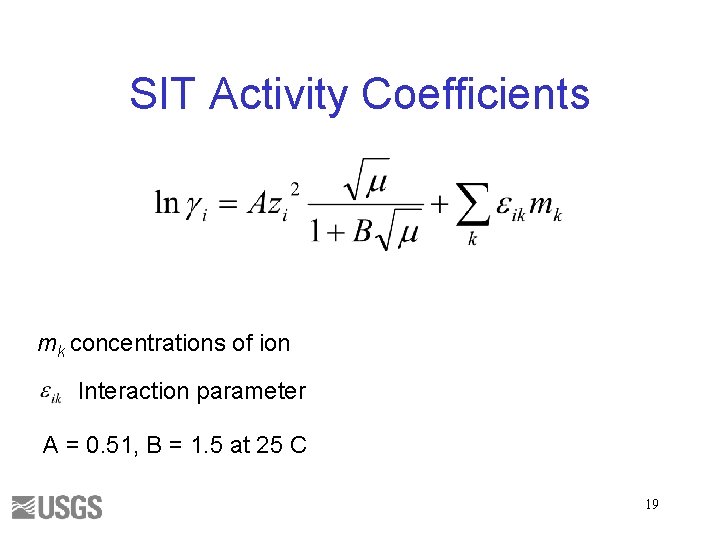
SIT Activity Coefficients mk concentrations of ion Interaction parameter A = 0. 51, B = 1. 5 at 25 C 19

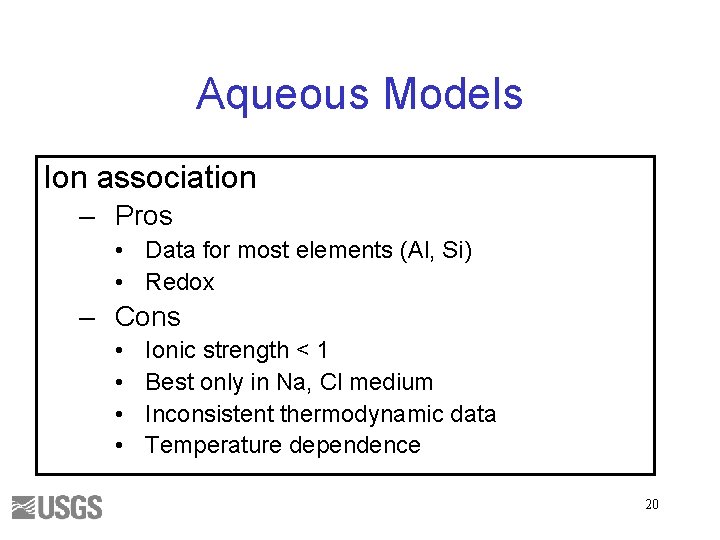
Aqueous Models Ion association – Pros • Data for most elements (Al, Si) • Redox – Cons • • Ionic strength < 1 Best only in Na, Cl medium Inconsistent thermodynamic data Temperature dependence 20

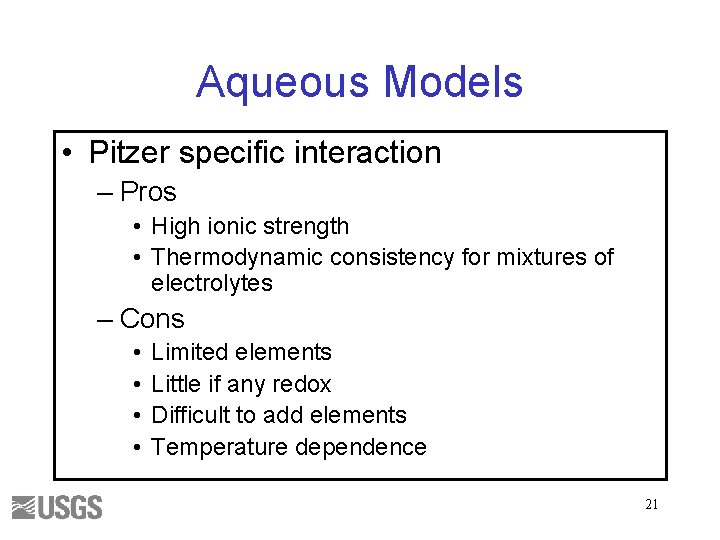
Aqueous Models • Pitzer specific interaction – Pros • High ionic strength • Thermodynamic consistency for mixtures of electrolytes – Cons • • Limited elements Little if any redox Difficult to add elements Temperature dependence 21

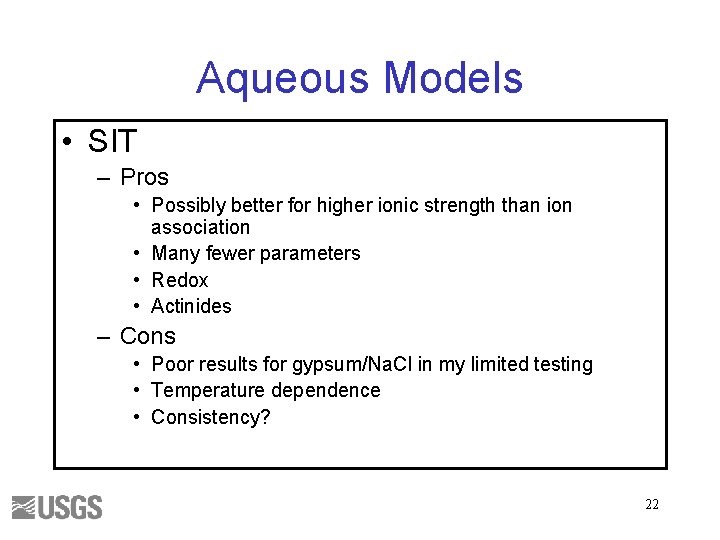
Aqueous Models • SIT – Pros • Possibly better for higher ionic strength than ion association • Many fewer parameters • Redox • Actinides – Cons • Poor results for gypsum/Na. Cl in my limited testing • Temperature dependence • Consistency? 22

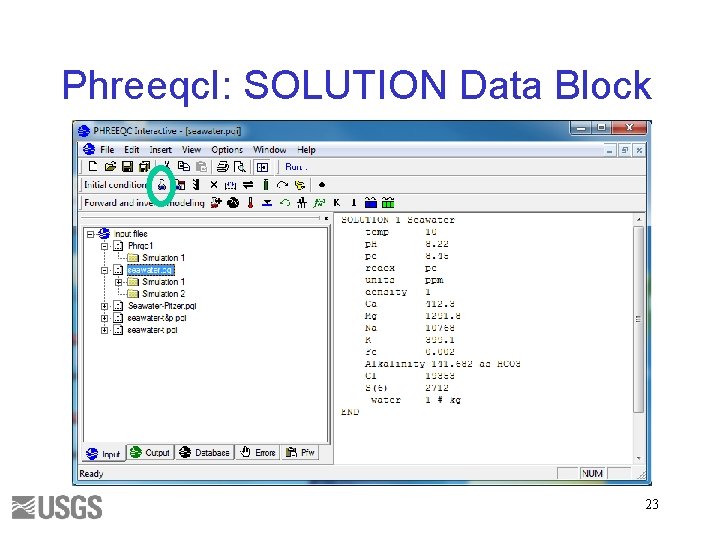
Phreeqc. I: SOLUTION Data Block 23

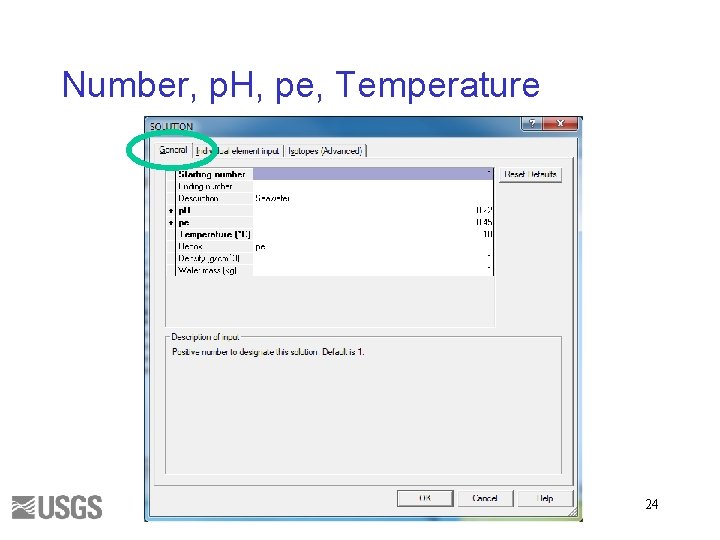
Number, p. H, pe, Temperature 24

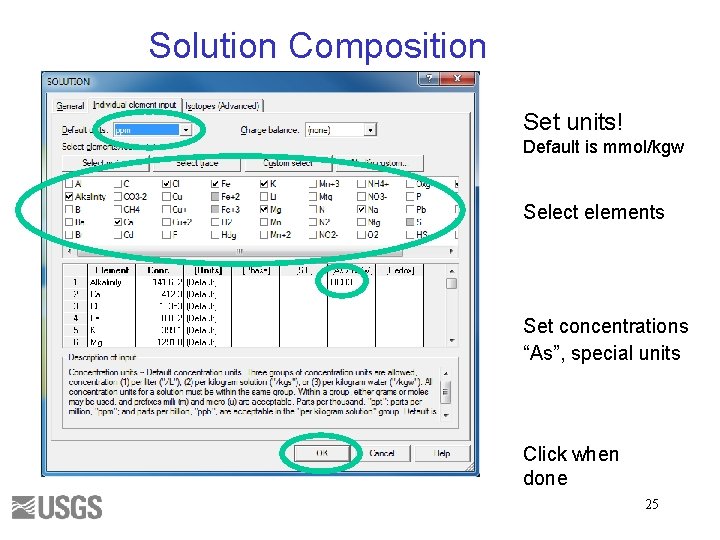
Solution Composition Set units! Default is mmol/kgw Select elements Set concentrations “As”, special units Click when done 25

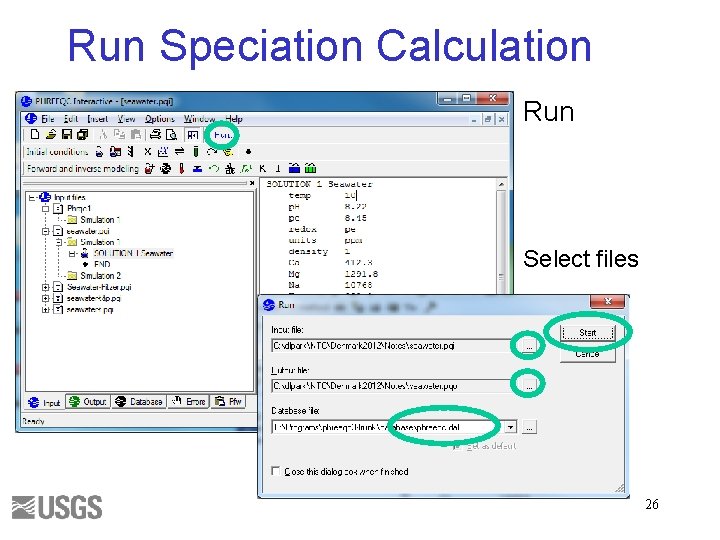
Run Speciation Calculation Run Select files 26

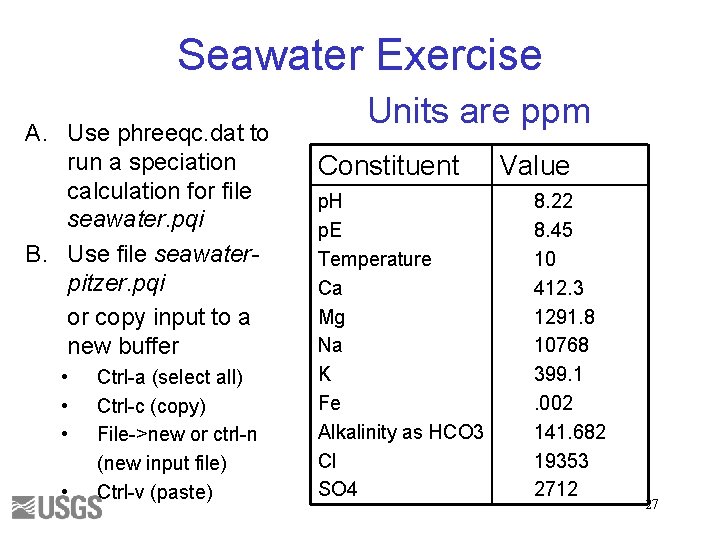
Seawater Exercise A. Use phreeqc. dat to run a speciation calculation for file seawater. pqi B. Use file seawaterpitzer. pqi or copy input to a new buffer • • Ctrl-a (select all) Ctrl-c (copy) File->new or ctrl-n (new input file) Ctrl-v (paste) Units are ppm Constituent p. H p. E Temperature Ca Mg Na K Fe Alkalinity as HCO 3 Cl SO 4 Value 8. 22 8. 45 10 412. 3 1291. 8 10768 399. 1. 002 141. 682 19353 2712 27

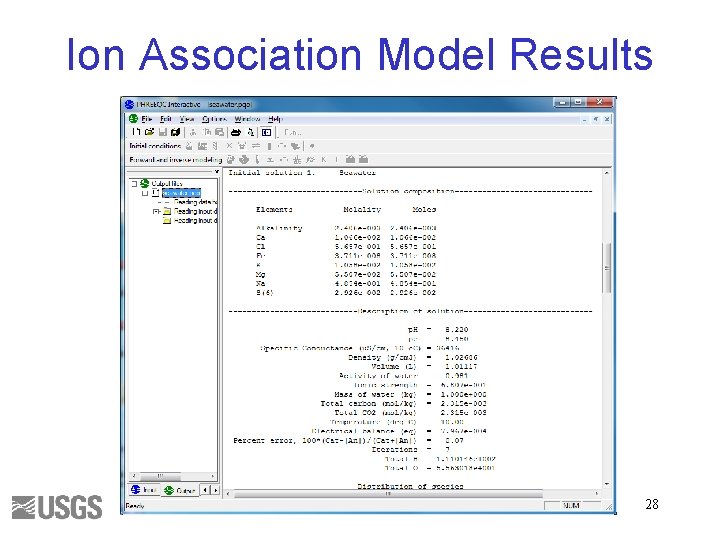
Ion Association Model Results 28

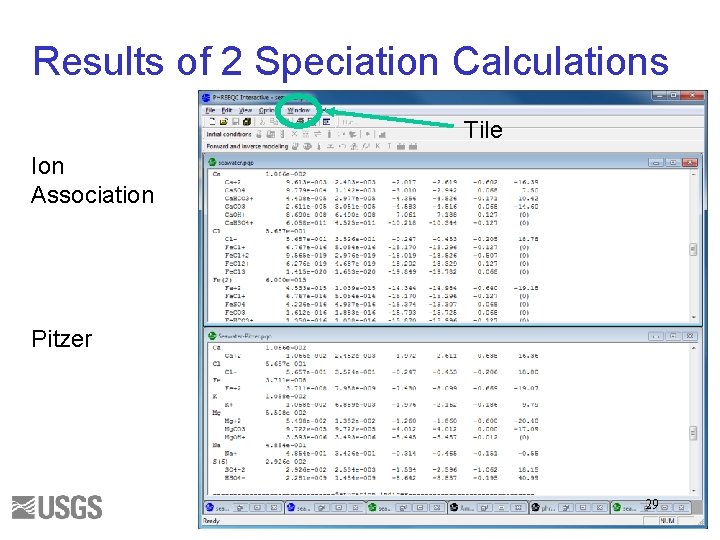
Results of 2 Speciation Calculations Tile Ion Association Pitzer 29

Questions 1. Write the mass-balance equation for calcium in seawater for each database. 2. What fraction of the total is Ca+2 ion for each database? 3. What fraction of the total is Fe+3 ion for each database? 4. What are the log activity and log activity coefficient of CO 3 -2 for each database? 5. What is the saturation index of calcite for each database? 30

Initial Solution 2. Answers () indicates molality 1 a. Ca(total)= 1. 066 e-2 = (Ca+2) + (Ca. SO 4) + (Ca. HCO 3+) + (Ca. CO 3) + (Ca. OH+) + (Ca. HSO 4+) 1 b. Ca(total) = 1. 066 e-2 = (Ca+2) + (Ca. CO 3) 2 a. 9. 5/10. 7 ~ 0. 95 2 b. 1. 063/1. 066 ~ 1. 0 3 a. 3. 509 e-019 / 3. 711 e-008 ~ 1 e-11 3 b. No Fe+3 ion. 4 a. log activity CO 3 -2 = -5. 099; log gamma CO 3 -2 = -0. 68 4 b. log activity CO 3 -2 = -5. 091; log gamma CO 3 -2 = -1. 09 5 a. SI(calcite) = 0. 76 5 b. SI(calcite) = 0. 70 31

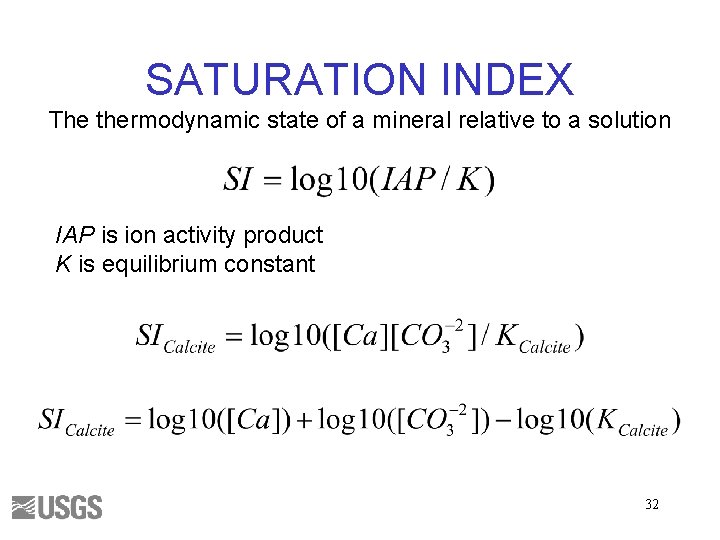
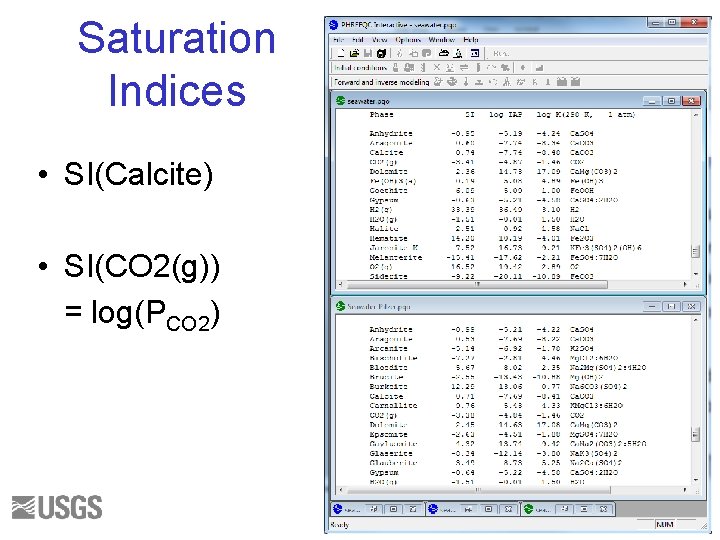
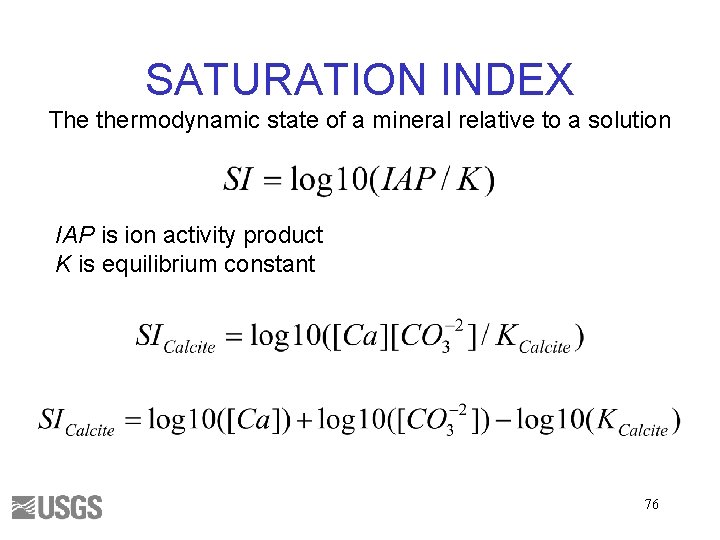
SATURATION INDEX The thermodynamic state of a mineral relative to a solution IAP is ion activity product K is equilibrium constant 32

SATURATION INDEX SI < 0, Mineral should dissolve SI > 0, Mineral should precipitate SI ~ 0, Mineral reacts fast enough to maintain equilibrium Maybe – Kinetics – Uncertainties 33

Rules for Saturation Indices • Mineral cannot dissolve if it is not present • If SI < 0 and mineral is present—the mineral could dissolve, but not precipitate • If SI > 0—the mineral could precipitate, but not dissolve • If SI ~ 0—the mineral could dissolve or precipitate to maintain equilibrium 34

Saturation Indices • SI(Calcite) • SI(CO 2(g)) = log(PCO 2) 35

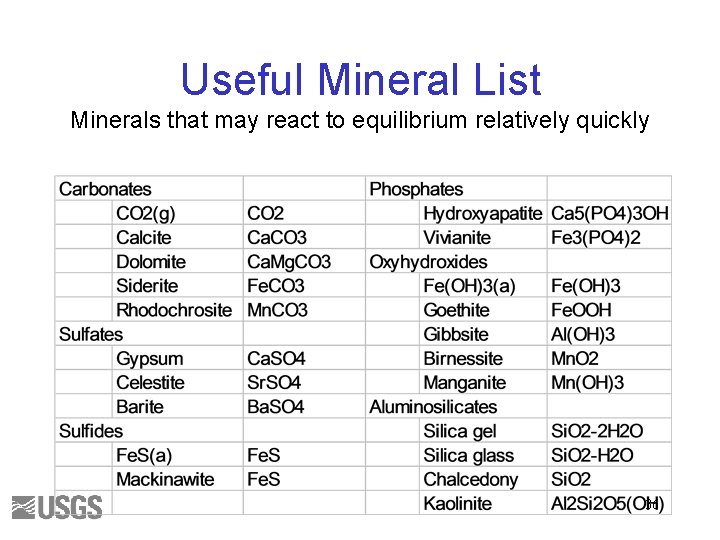
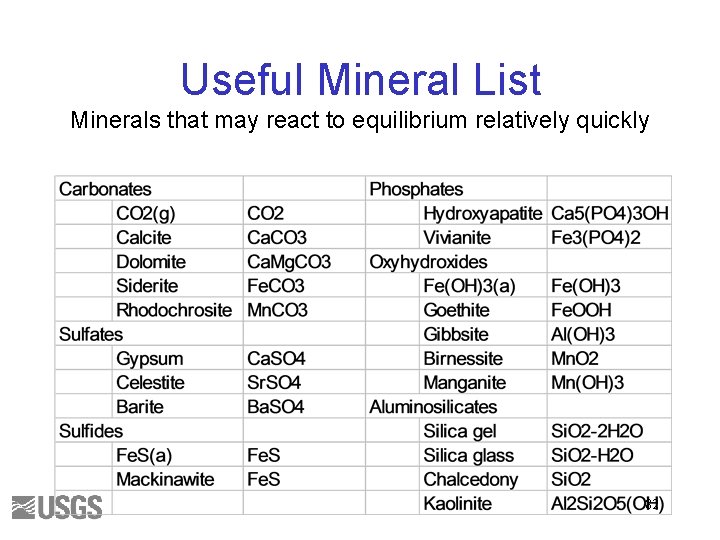
Useful Mineral List Minerals that may react to equilibrium relatively quickly 36

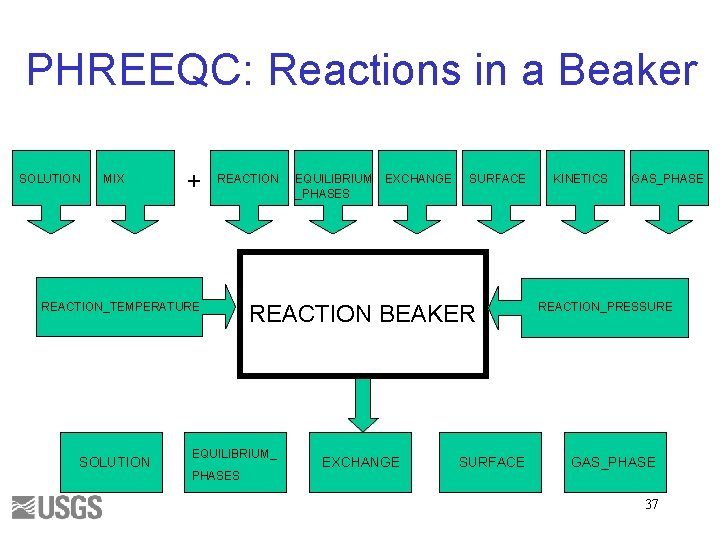
PHREEQC: Reactions in a Beaker SOLUTION MIX + REACTION_TEMPERATURE SOLUTION EXCHANGE SURFACE REACTION BEAKER EQUILIBRIUM_ PHASES EQUILIBRIUM _PHASES EXCHANGE SURFACE KINETICS GAS_PHASE REACTION_PRESSURE GAS_PHASE 37

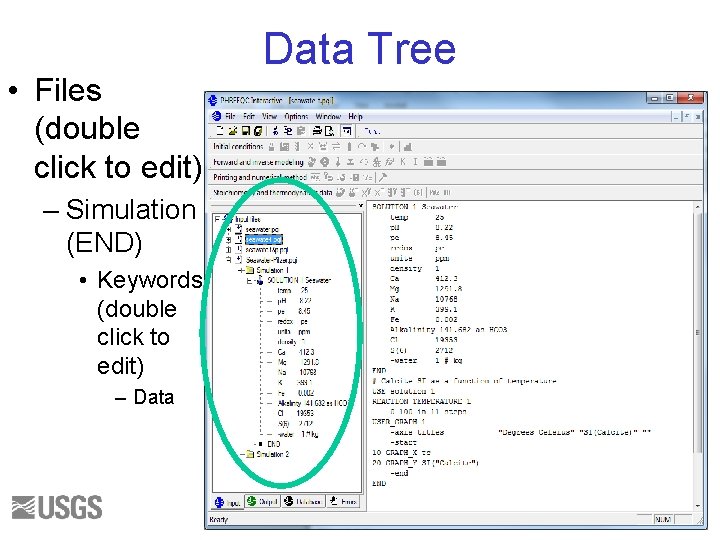
• Files (double click to edit) Data Tree – Simulation (END) • Keywords (double click to edit) – Data 38

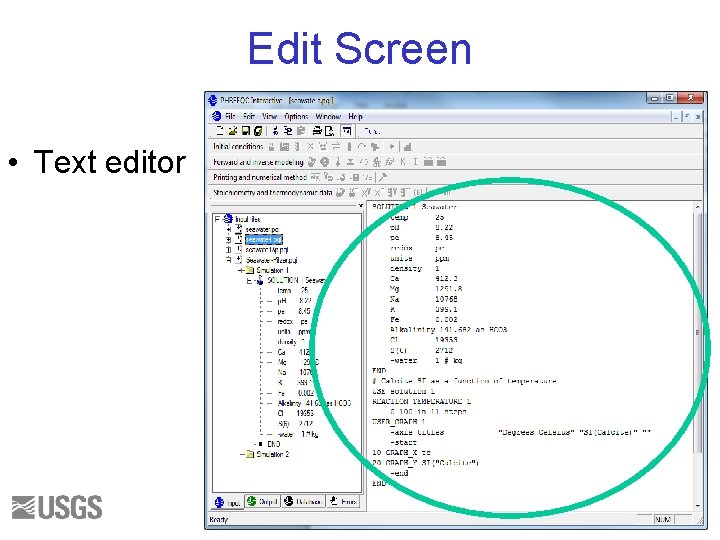
Edit Screen • Text editor 39

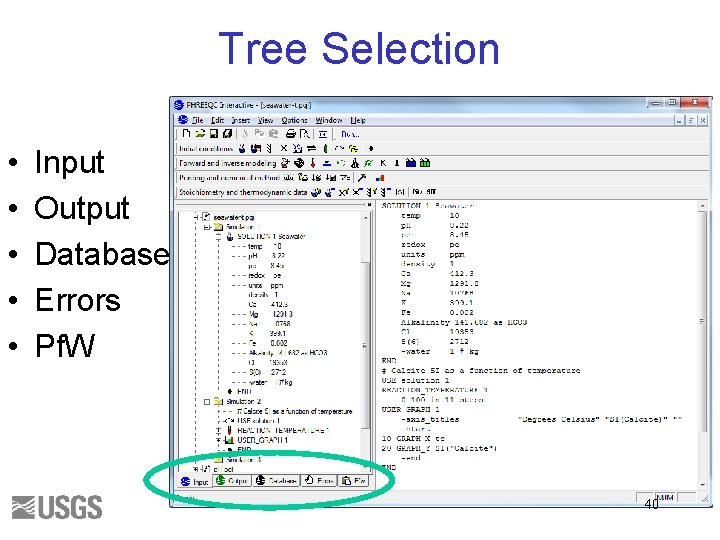
Tree Selection • • • Input Output Database Errors Pf. W 40

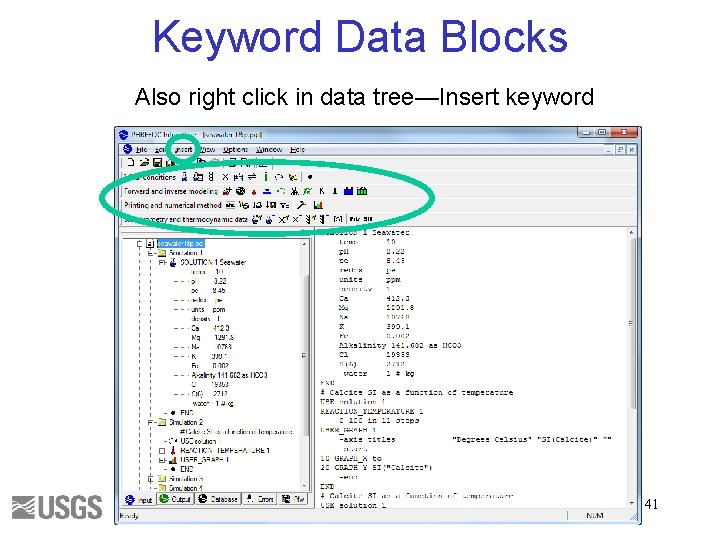
Keyword Data Blocks Also right click in data tree—Insert keyword 41

Pf. W Style 42

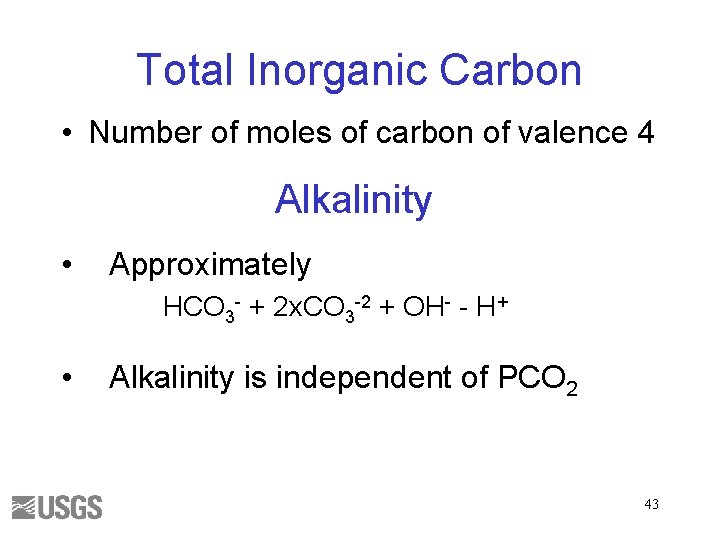
Total Inorganic Carbon • Number of moles of carbon of valence 4 Alkalinity • Approximately HCO 3 - + 2 x. CO 3 -2 + OH- - H+ • Alkalinity is independent of PCO 2 43

SOLUTION_SPREAD 44

Carbon and Alkalinity solution_spread. pqi SOLUTION_SPREAD SELECTED_OUTPUT USER_GRAPH 45

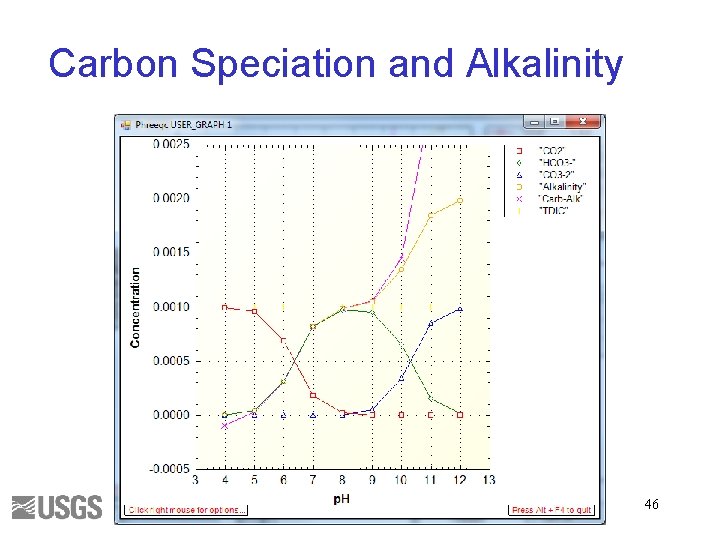
Carbon Speciation and Alkalinity 46

p. H and pe Keywords SOLUTION—Solution composition END—End of a simulation USE—Reactant to add to beaker REACTION—Specified moles of a reaction USER_GRAPH—Charting 47

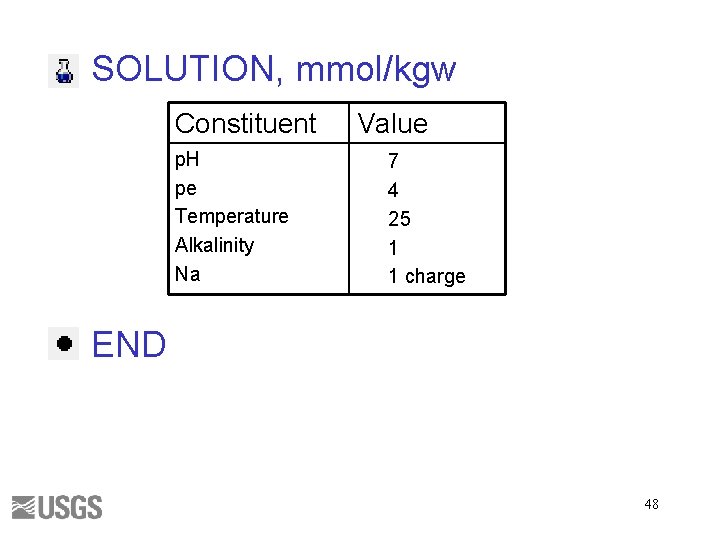
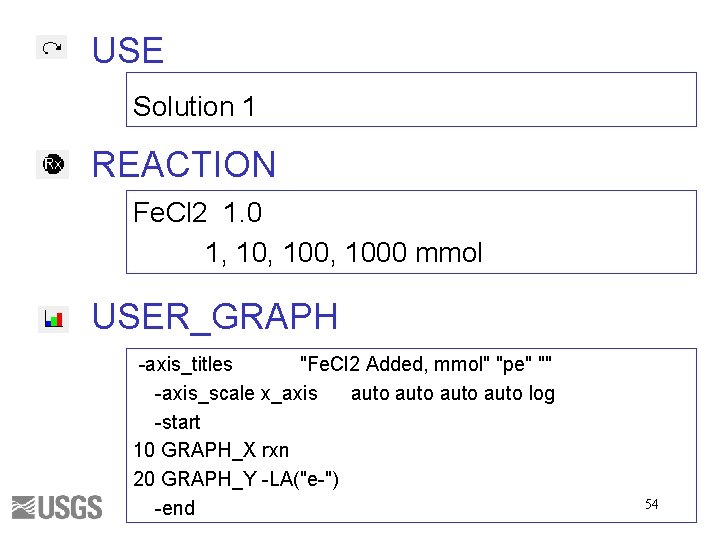
SOLUTION, mmol/kgw Constituent p. H pe Temperature Alkalinity Na Value 7 4 25 1 1 charge END 48

USE Solution 1 REACTION CO 2 1. 0 1, 100, 1000 mmol USER_GRAPH -axis_titles "CO 2 Added, mmol" "p. H" "Alkalinity" -axis_scale x_axis auto log -axis_scale sy_axis 0 0. 002 -start 10 GRAPH_X rxn 20 GRAPH_Y -LA("H+") 30 GRAPH_SY ALK -end 49

Input file p. H. pqi SOLUTION 1 temp 25 p. H 7 pe 4 redox pe units mmol/kgw density 1 Alkalinity 1 Na 1 charge -water 1 # kg END USE solution 1 REACTION 1 CO 2 1 1 10 1000 millimoles USER_GRAPH 1 -axis_titles "CO 2 Added, mmol" "p. H" "Alkalinity" -axis_scale x_axis auto log -axis_scale sy_axis 0 0. 002 -start 10 GRAPH_X rxn 20 GRAPH_Y -LA("H+") 30 GRAPH_SY ALK -end END 50

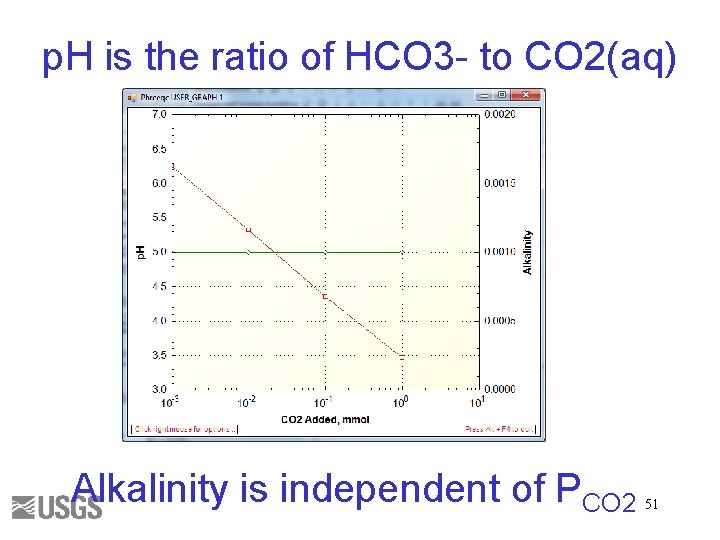
p. H is the ratio of HCO 3 - to CO 2(aq) Alkalinity is independent of PCO 2 51
![What is p H p H 6 3 logHCO 3 CO 2 What is p. H? p. H = 6. 3 + log[(HCO 3 -)/(CO 2)]](https://slidetodoc.com/presentation_image/4d6b98f8dda8f663eadd138445bc6fb3/image-52.jpg)
What is p. H? p. H = 6. 3 + log[(HCO 3 -)/(CO 2)] p. H = 10. 3 + log[(CO 3 -2)/(HCO 3 -)] p. H = log. K + log[(PO 4 -3)/(HPO 4 -2)] Questions 1. How does the p. H change when CO 2 degasses during an alkalinity titration? 2. How does p. H change when plankton respire CO 2? 3. How does p. H change when calcite dissolves? 52

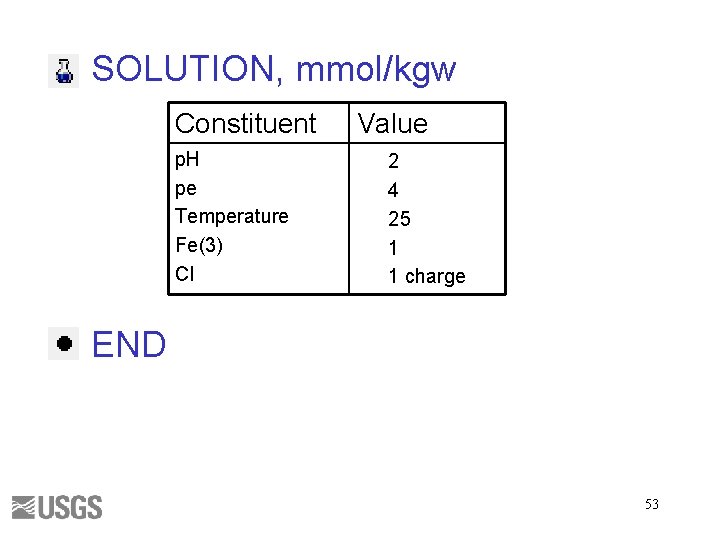
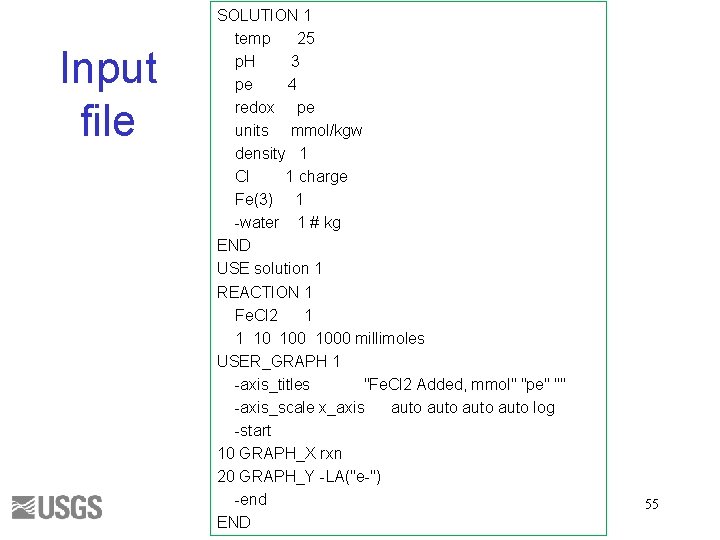
SOLUTION, mmol/kgw Constituent p. H pe Temperature Fe(3) Cl Value 2 4 25 1 1 charge END 53

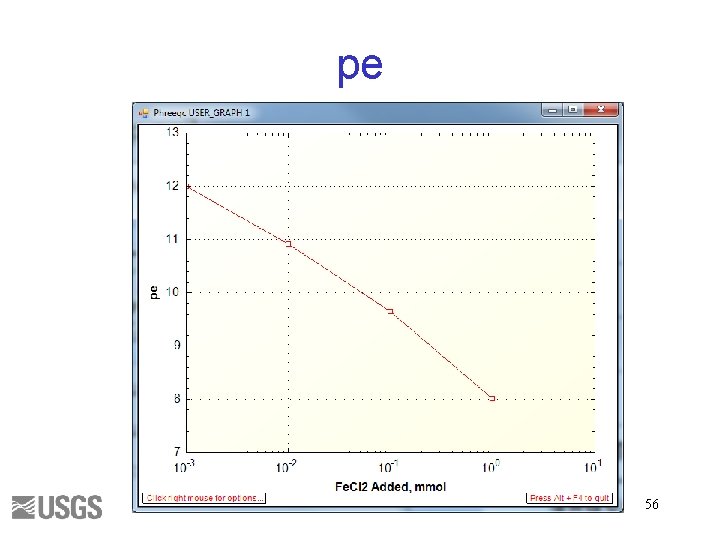
USE Solution 1 REACTION Fe. Cl 2 1. 0 1, 100, 1000 mmol USER_GRAPH -axis_titles "Fe. Cl 2 Added, mmol" "pe" "" -axis_scale x_axis auto log -start 10 GRAPH_X rxn 20 GRAPH_Y -LA("e-") -end 54

Input file SOLUTION 1 temp 25 p. H 3 pe 4 redox pe units mmol/kgw density 1 Cl 1 charge Fe(3) 1 -water 1 # kg END USE solution 1 REACTION 1 Fe. Cl 2 1 1 10 1000 millimoles USER_GRAPH 1 -axis_titles "Fe. Cl 2 Added, mmol" "pe" "" -axis_scale x_axis auto log -start 10 GRAPH_X rxn 20 GRAPH_Y -LA("e-") -end END 55

pe 56
![What is pe Fe2 Fe3 epe log Fe3Fe2 13 What is pe? Fe+2 = Fe+3 + epe = log( [Fe+3]/[Fe+2] ) + 13](https://slidetodoc.com/presentation_image/4d6b98f8dda8f663eadd138445bc6fb3/image-57.jpg)
What is pe? Fe+2 = Fe+3 + epe = log( [Fe+3]/[Fe+2] ) + 13 HS- + 4 H 2 O = SO 4 -2 + 9 H+ + 8 epe = log( [SO 4 -2]/[HS-] ) – 9/8 p. H + 4. 21 N 2 + 6 H 2 O = 2 NO 3 - + 12 H+ + 10 epe = 0. 1 log( [NO 3 -]2/[N 2] ) – 1. 2 p. H + 20. 7 pe = 16. 9 Eh, Eh in volts (platinum electrode measurement) 57

Redox and pe in SOLUTION Data Blocks • When do you need pe for SOLUTION? – To distribute total concentration of a redox element among redox states [e. g. Fe to Fe(2) and Fe(3)] – A few saturation indices with e- in dissociation reactions • Pyrite • Native sulfur • Manganese oxides • Can use a redox couple Fe(2)/Fe(3) in place of pe • Rarely, pe = 16. 9 Eh. (25 C and Eh in Volts). • pe options can only be applied to speciation calculations; thermodynamic pe is used for all other calculations 58

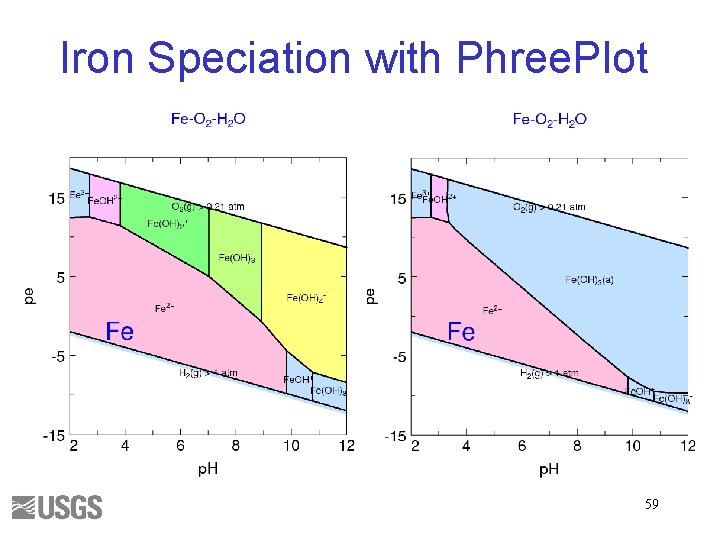
Iron Speciation with Phree. Plot 59

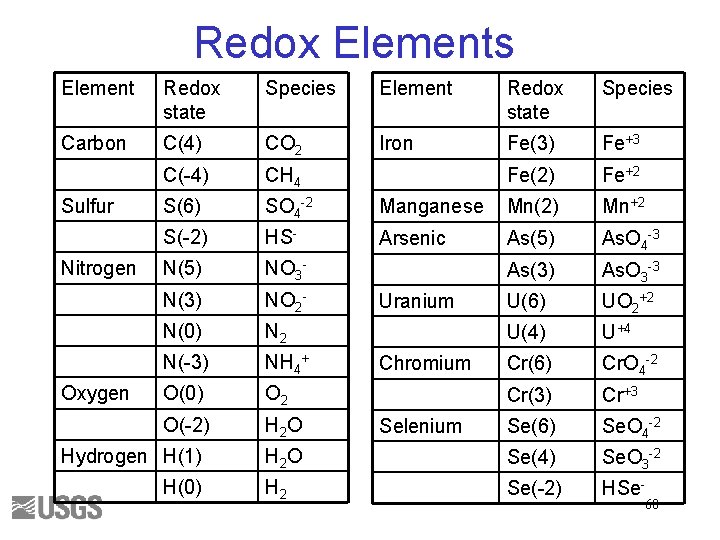
Redox Elements Element Redox state Species Carbon C(4) CO 2 Iron Fe(3) Fe+3 C(-4) CH 4 Fe(2) Fe+2 S(6) SO 4 -2 Manganese Mn(2) Mn+2 S(-2) HS- Arsenic As(5) As. O 4 -3 N(5) NO 3 - As(3) As. O 3 -3 N(3) NO 2 - U(6) UO 2+2 N(0) N 2 U(4) U+4 N(-3) NH 4+ Cr(6) Cr. O 4 -2 O(0) O 2 Cr(3) Cr+3 O(-2) H 2 O Se(6) Se. O 4 -2 H 2 O Se(4) Se. O 3 -2 H 2 Se(-2) HSe- Sulfur Nitrogen Oxygen Hydrogen H(1) H(0) Uranium Chromium Selenium 60

Seawater Initial Solution Fe total was entered. How were Fe(3) and Fe(2) concentrations calculated? For initial solutions For “reactions” 61

Final thoughts on pe • pe sets ratio of redox states • Some redox states are measured directly: – – NO 3 -, NO 2 -, NH 3, N 2(aq) SO 4 -2, HSO 2(aq) Sometimes Fe, As • Others can be assumed: – – – Fe, always Fe(2) except at low p. H Mn, always Mn(2) As, consider other redox elements Se, consider other redox elements U, probably U(6) V, probably V(5) 62

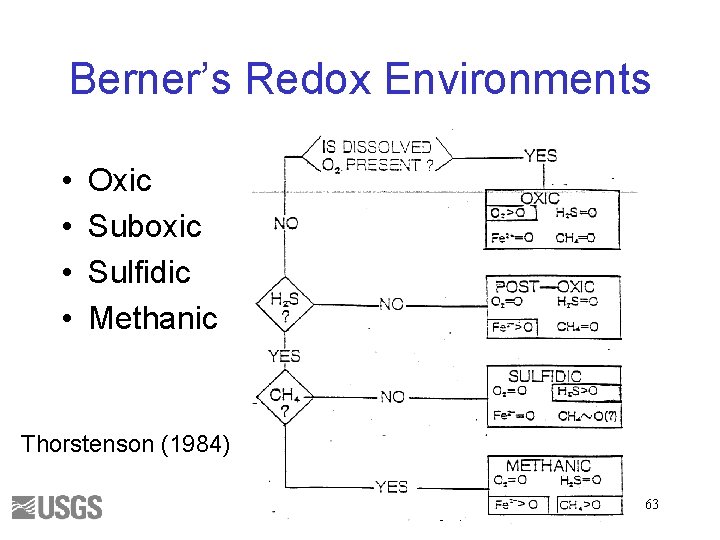
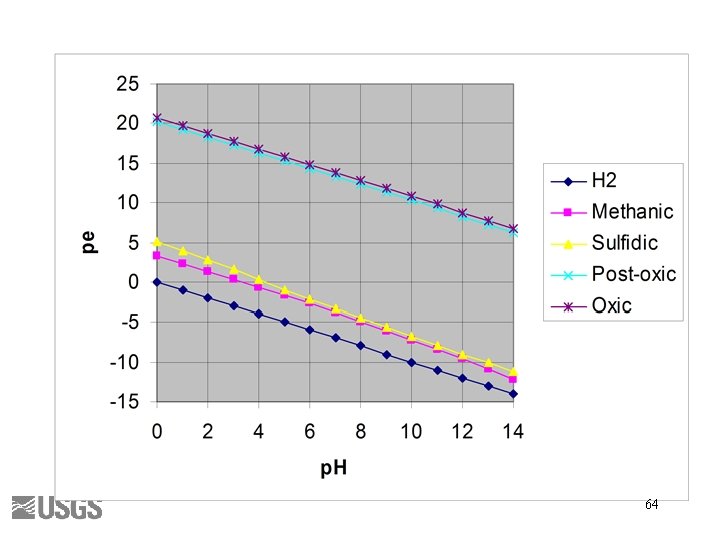
Berner’s Redox Environments • • Oxic Suboxic Sulfidic Methanic Thorstenson (1984) 63

64

Parkhurst and others (1996) 65

Summary on Solutions • • • Aqueous model SOLUTION p. H—Ratio of HCO 3 - to CO 2 pe—Ratio of oxidized to reduced state Saturation indices 66

Reaction Simulations • SOLUTION, SOLUTION_SPREAD, MIX, USE solution, or USE mix Equilibrium EQUILIBRIUM_PHASES EXCHANGE SURFACE SOLID_SOLUTION GAS_PHASE REACTION_TEMPERATURE REACTION_PRESSURE Nonequilibrium • END KINETICS REACTION 67

Keywords SOLUTION END USE REACTION_TEMPERATURE USER_GRAPH REACTION_PRESSURE 68

Plot the SI of Calcite with Temperature Seawater-t&p. pqi 69

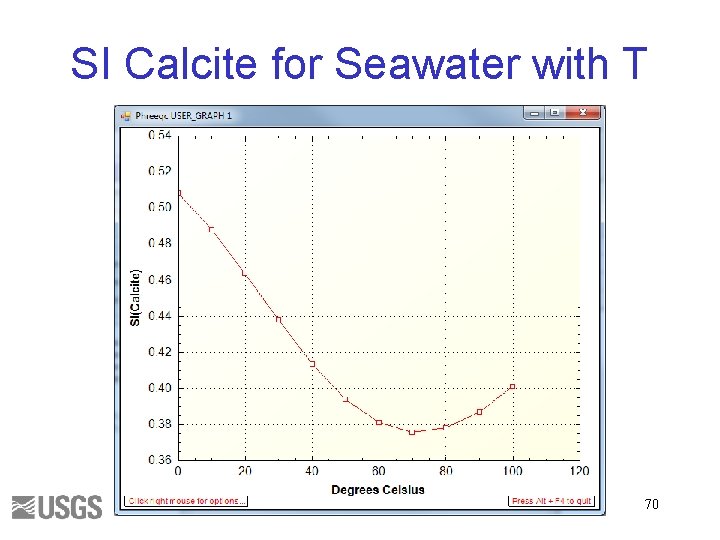
SI Calcite for Seawater with T 70

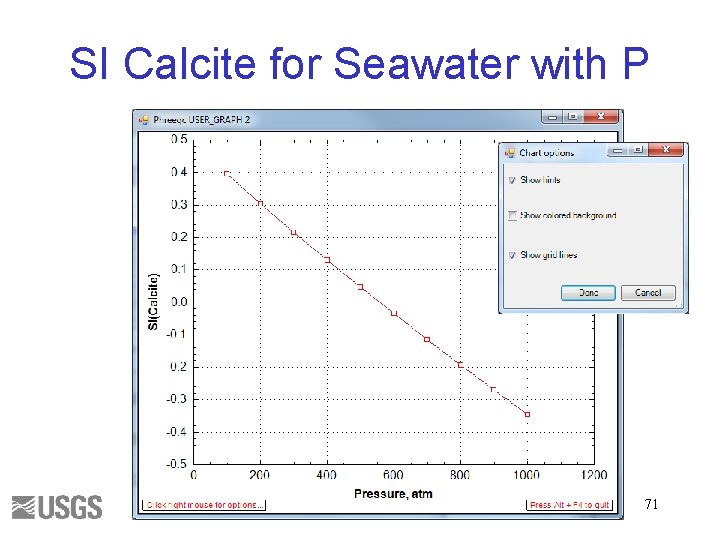
SI Calcite for Seawater with P 71

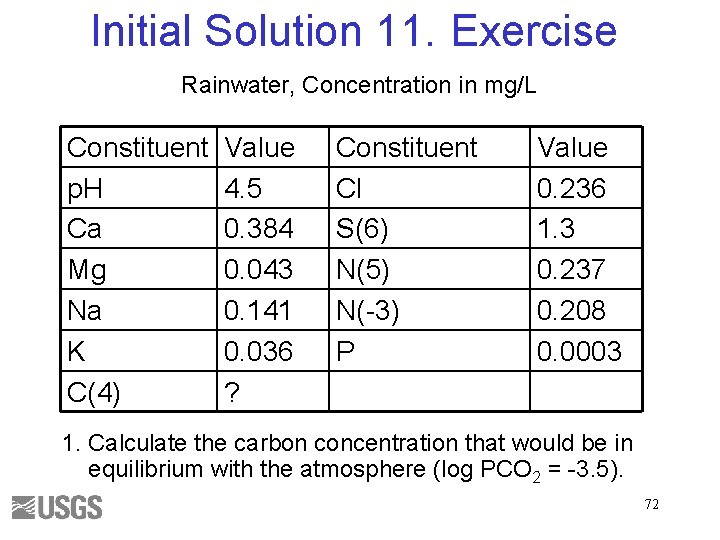
Initial Solution 11. Exercise Rainwater, Concentration in mg/L Constituent p. H Ca Mg Na K C(4) Value 4. 5 0. 384 0. 043 0. 141 0. 036 ? Constituent Cl S(6) N(5) N(-3) P Value 0. 236 1. 3 0. 237 0. 208 0. 0003 1. Calculate the carbon concentration that would be in equilibrium with the atmosphere (log PCO 2 = -3. 5). 72

Initial Solution 11. Answer 1. Calculate the carbon concentration that would be in equilibrium with the atmosphere (log PCO 2 = -3. 5). 1. 1 e-5 mol C per kilogram water 73

Initial Solution 12. Exercise 1. Calculate the p. H and TDIC of a solution in equilibrium with the PCO 2 of air (10 -3. 5) at 25 C. 2. Calculate the p. H and TDIC of a solution in equilibrium with a soil-zone PCO 2 of 10 -2. 0 at 25 C. 3. Calculate the p. H and TDIC of a solution in equilibrium with a soil-zone PCO 2 of 10 -2. 0 at 10 C. 74

Initial Solution 12. Answers 1. p. H = 5. 66, TDIC = 13 umol/kgw 2. p. H = 4. 91, TDIC = 353 umol/kgw 3. p. H = 4. 87, TDIC = 552 umol/kgw 75

SATURATION INDEX The thermodynamic state of a mineral relative to a solution IAP is ion activity product K is equilibrium constant 76

SATURATION INDEX SI < 0, Mineral should dissolve SI > 0, Mineral should precipitate SI ~ 0, Mineral reacts fast enough to maintain equilibrium Maybe – Kinetics – Uncertainties 77

Rules for Saturation Indices • Mineral cannot dissolve if it is not present • If SI < 0 and mineral is present—the mineral could dissolve, but not precipitate • If SI > 0—the mineral could precipitate, but not dissolve • If SI ~ 0—the mineral could dissolve or precipitate to maintain equilibrium 78

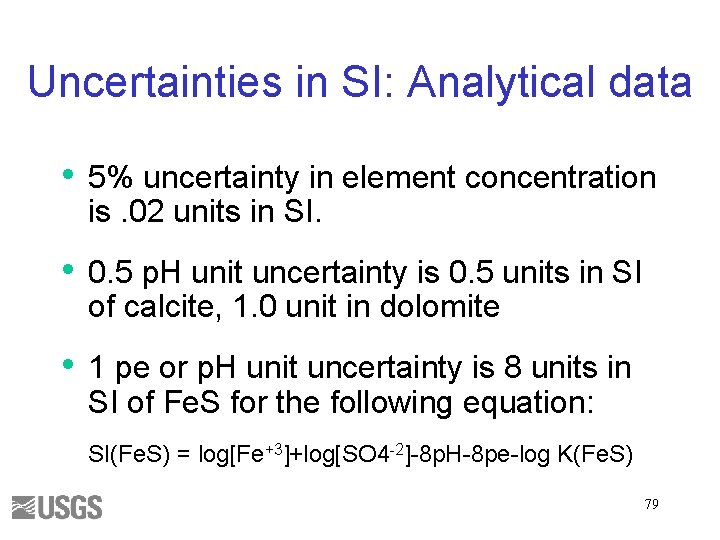
Uncertainties in SI: Analytical data • 5% uncertainty in element concentration is. 02 units in SI. • 0. 5 p. H unit uncertainty is 0. 5 units in SI of calcite, 1. 0 unit in dolomite • 1 pe or p. H unit uncertainty is 8 units in SI of Fe. S for the following equation: SI(Fe. S) = log[Fe+3]+log[SO 4 -2]-8 p. H-8 pe-log K(Fe. S) 79

Uncertainties in SI: Equation • Much smaller uncertainty for SI(Fe. S) with the following equation : SI(Fe. S) = log[Fe+2]+log[HS-]+p. H-log K(Fe. S) • For minerals with redox elements, uncertainties are much smaller if the valence states of the elements in solution are measured. 80

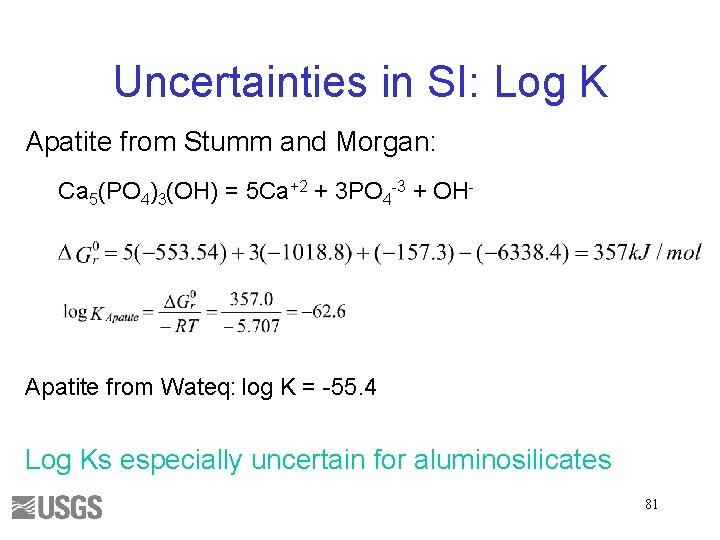
Uncertainties in SI: Log K Apatite from Stumm and Morgan: Ca 5(PO 4)3(OH) = 5 Ca+2 + 3 PO 4 -3 + OH- Apatite from Wateq: log K = -55. 4 Log Ks especially uncertain for aluminosilicates 81

Useful Mineral List Minerals that may react to equilibrium relatively quickly 82

Initial Solution 13. Exercise Examine solution compositions in spreadsheet “solution_spread. xls”. Calculate saturation indices using phreeqc. dat. Try out Run. Phreeqc macro or copy/paste into Phreeqc. I. What can you infer about the hydrologic setting, mineralogy, and possible reactions for these waters? 83

Solution_spread. xls + is 13. xls 84

Summary Aqueous speciation model – Mole-balance equations—Sum of species containing Ca equals total analyzed Ca – Aqueous mass-action equations—Activity of products over reactants equal a constant Activity coefficient model – • • – Ion association with individual activity coefficients Pitzer specific interaction approach SI=log(IAP/K) 85

Summary SOLUTION and SOLUTION _SPREAD – – – Units p. H—ratio of HCO 3/CO 2 pe—ratio of oxidized/reduced valence states Charge balance Phase boundaries • Saturation indices – Uncertainties – Useful minerals • Identify potential reactants 86

87

88
 Markus pitzer
Markus pitzer Dr michael pitzer
Dr michael pitzer Michael pitzer
Michael pitzer Dan pitzer
Dan pitzer Reactions in aqueous solutions
Reactions in aqueous solutions Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Modern chemistry chapter 13
Modern chemistry chapter 13 Aqueous solutions module
Aqueous solutions module Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Freezing point chapter 13
Freezing point chapter 13 General properties of aqueous solutions
General properties of aqueous solutions Aqueous solution practice problems
Aqueous solution practice problems Electrical conductivity of aqueous solutions
Electrical conductivity of aqueous solutions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Are aqueous solutions homogeneous mixtures
Are aqueous solutions homogeneous mixtures Are aqueous solutions homogeneous mixtures
Are aqueous solutions homogeneous mixtures Solubility guidelines for aqueous solutions
Solubility guidelines for aqueous solutions Chapter 4 reactions in aqueous solutions
Chapter 4 reactions in aqueous solutions Chapter 4 reactions in aqueous solutions worksheet answers
Chapter 4 reactions in aqueous solutions worksheet answers Which solution
Which solution Ion dipolo
Ion dipolo Fuerzas dipolo dipolo ejemplos
Fuerzas dipolo dipolo ejemplos Fuerzas dipolo dipolo ejemplos
Fuerzas dipolo dipolo ejemplos London dispersion
London dispersion Dr. erukhimova
Dr. erukhimova Hittorf cell
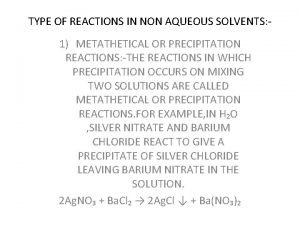
Hittorf cell Types of reaction in non aqueous solvents
Types of reaction in non aqueous solvents For clarity of aqueous extract test container autoclaved at
For clarity of aqueous extract test container autoclaved at Urutan kelarutan perak halida dalam pelarut air
Urutan kelarutan perak halida dalam pelarut air Inorganic non aqueous solvents
Inorganic non aqueous solvents S solid liquid or gas
S solid liquid or gas Additional aspects of aqueous equilibria
Additional aspects of aqueous equilibria Chapter 15 water and aqueous systems
Chapter 15 water and aqueous systems Water and aqueous systems chapter 15 answers
Water and aqueous systems chapter 15 answers Do pickles conduct electricity
Do pickles conduct electricity Additional aspects of aqueous equilibria
Additional aspects of aqueous equilibria Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry An artificial cell consisting of an aqueous solution
An artificial cell consisting of an aqueous solution Aqueous acid base titration
Aqueous acid base titration Heterogeneous aqueous systems
Heterogeneous aqueous systems Homogeneous aqueous systems
Homogeneous aqueous systems Is water aqueous
Is water aqueous Liquid so2 as a non aqueous solvent
Liquid so2 as a non aqueous solvent Impression compound manipulation
Impression compound manipulation Elastomeric impression materials examples
Elastomeric impression materials examples Chemsheets reactions of halogenoalkanes 2
Chemsheets reactions of halogenoalkanes 2 Aqueous equilibria
Aqueous equilibria Halogenoalkane to alcohol mechanism
Halogenoalkane to alcohol mechanism Derive henderson equation
Derive henderson equation Brf3 is a protic solvent
Brf3 is a protic solvent Non aqueous media
Non aqueous media Birdshot retinochoroidopathy
Birdshot retinochoroidopathy Aqueous humor
Aqueous humor Aqueous ammonia
Aqueous ammonia Eye diagram pearson
Eye diagram pearson Conjunctivitis differential diagnosis
Conjunctivitis differential diagnosis Titrate
Titrate Spiral organ of corti
Spiral organ of corti Monophasic liquid example
Monophasic liquid example Aldol reaction
Aldol reaction Chapter 15 water and aqueous systems
Chapter 15 water and aqueous systems Aqueous
Aqueous Assume that an aqueous solution of a cation
Assume that an aqueous solution of a cation Chapter 15 water and aqueous systems
Chapter 15 water and aqueous systems Why study thermodynamics
Why study thermodynamics Thermodynamics webquest
Thermodynamics webquest Rubbing hands together energy
Rubbing hands together energy Property tables thermodynamics
Property tables thermodynamics Internal energy formula thermodynamics
Internal energy formula thermodynamics Nozzle and diffuser
Nozzle and diffuser First law of thermodynamics for open system
First law of thermodynamics for open system Thermodynamics deals with
Thermodynamics deals with Stat mech
Stat mech Mech
Mech Zeroth law of thermodynamics definition
Zeroth law of thermodynamics definition Third law of thermodynamics derivation
Third law of thermodynamics derivation Zeroth law of thermodynamics statement
Zeroth law of thermodynamics statement Engineering thermodynamics
Engineering thermodynamics First law of thermodynamics
First law of thermodynamics Cyclic process thermodynamics
Cyclic process thermodynamics Residual properties in thermodynamics
Residual properties in thermodynamics Laws of thermodynamics simple
Laws of thermodynamics simple Statistical thermodynamics is a study of
Statistical thermodynamics is a study of Free energy
Free energy In a spontaneous irreversible process
In a spontaneous irreversible process Isentropic compressor
Isentropic compressor Thermodynamics 1 formula sheet
Thermodynamics 1 formula sheet Thermodynamic equilibrium definition
Thermodynamic equilibrium definition Cop of refrigerator
Cop of refrigerator