The First Law of Thermodynamics The Law of









- Slides: 18

The First Law of Thermodynamics The Law of Conservation of Energy

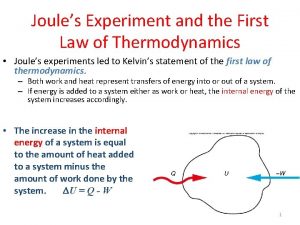
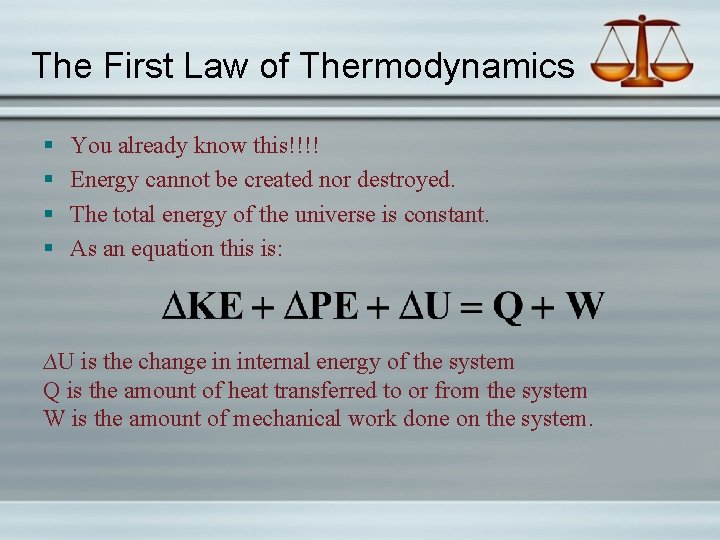
The First Law of Thermodynamics § § You already know this!!!! Energy cannot be created nor destroyed. The total energy of the universe is constant. As an equation this is: ∆U is the change in internal energy of the system Q is the amount of heat transferred to or from the system W is the amount of mechanical work done on the system.

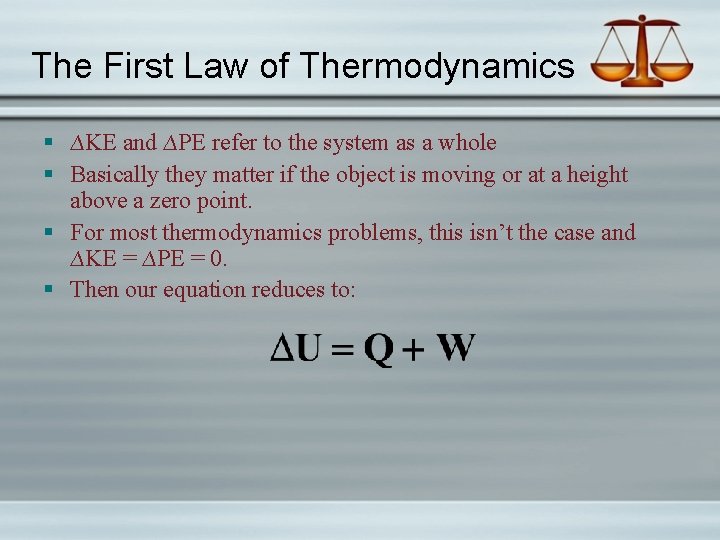
The First Law of Thermodynamics § ∆KE and ∆PE refer to the system as a whole § Basically they matter if the object is moving or at a height above a zero point. § For most thermodynamics problems, this isn’t the case and ∆KE = ∆PE = 0. § Then our equation reduces to:

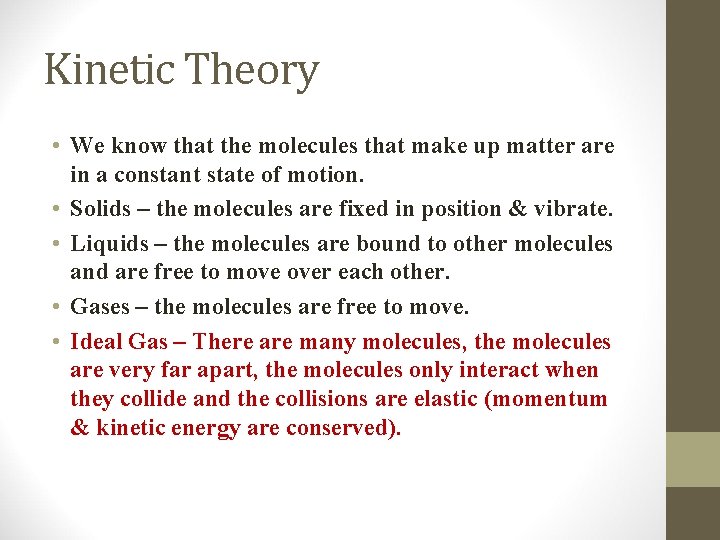
Kinetic Theory • We know that the molecules that make up matter are in a constant state of motion. • Solids – the molecules are fixed in position & vibrate. • Liquids – the molecules are bound to other molecules and are free to move over each other. • Gases – the molecules are free to move. • Ideal Gas – There are many molecules, the molecules are very far apart, the molecules only interact when they collide and the collisions are elastic (momentum & kinetic energy are conserved).

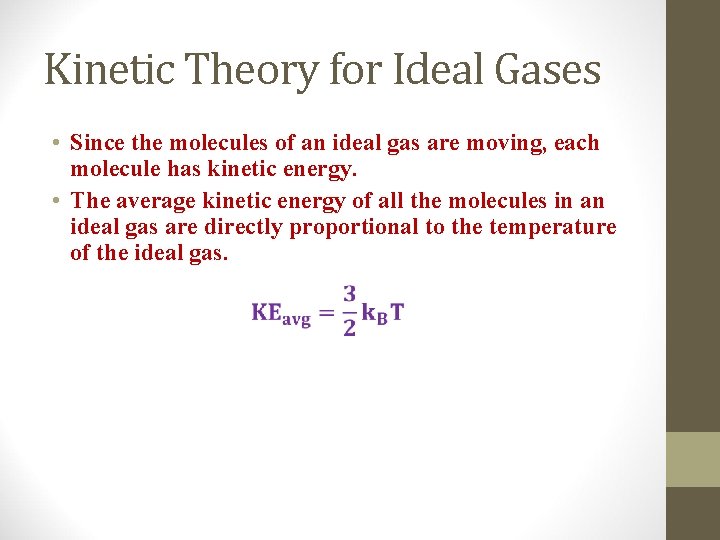
Kinetic Theory for Ideal Gases • Since the molecules of an ideal gas are moving, each molecule has kinetic energy. • The average kinetic energy of all the molecules in an ideal gas are directly proportional to the temperature of the ideal gas.

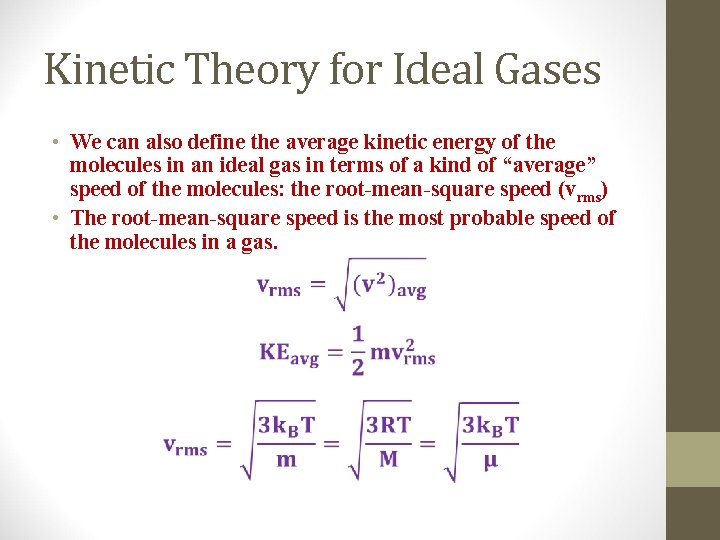
Kinetic Theory for Ideal Gases • We can also define the average kinetic energy of the molecules in an ideal gas in terms of a kind of “average” speed of the molecules: the root-mean-square speed (vrms) • The root-mean-square speed is the most probable speed of the molecules in a gas.

Internal Energy The sum total of all the energy of all the molecules in an object. v Very much related to the temperature of a substance. v The internal energy of an ideal gas is the total kinetic energy of all of the molecules in the gas. v

Internal Energy of an Ideal Gas In terms of the number of molecules (N) and Boltzmann’s Constant: In terms of the number of moles of the gas (n) and the Universal Gas Constant:

Internal Energy of an Ideal Gas The change in internal energy then is: In terms of the number of molecules (N) and Boltzmann’s Constant: In terms of the number of moles of the gas (n) and the Universal Gas Constant:

Heat Transfer The transfer of energy from one object to another BECAUSE of difference in temperature. Heat always flows from high temperature to low temperature. Heat was measured in calories (cal) A calorie is the amount of heat necessary to raise the temperature of 1 gram of water 1 o. C.

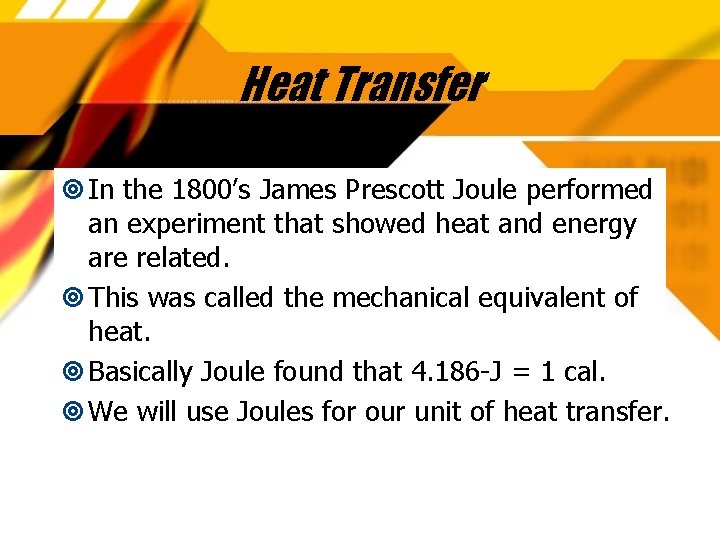
Heat Transfer In the 1800’s James Prescott Joule performed an experiment that showed heat and energy are related. This was called the mechanical equivalent of heat. Basically Joule found that 4. 186 -J = 1 cal. We will use Joules for our unit of heat transfer.

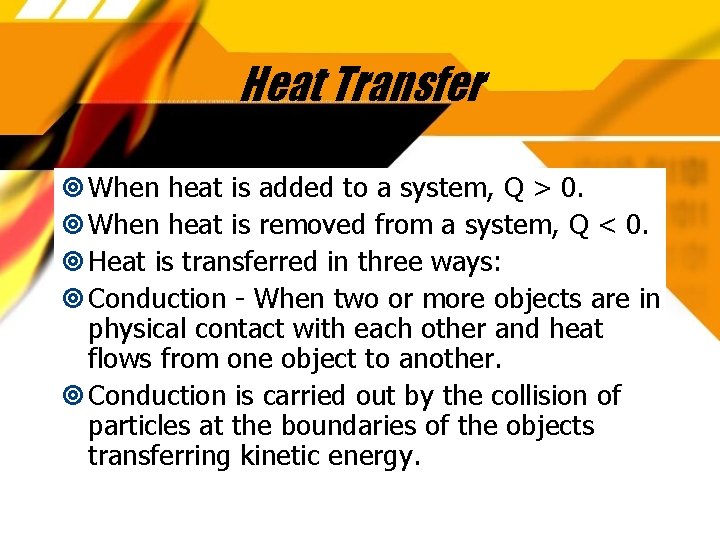
Heat Transfer When heat is added to a system, Q > 0. When heat is removed from a system, Q < 0. Heat is transferred in three ways: Conduction - When two or more objects are in physical contact with each other and heat flows from one object to another. Conduction is carried out by the collision of particles at the boundaries of the objects transferring kinetic energy.

Heat Transfer Convection - When heat flows through the mass movement of molecules from one place to another. When air near the Earth’s surface is heated by the ground it rises. When this air rises it cools and then sinks again. These are called convection currents. Radiation - Substances absorb light, microwaves, Ultraviolet Rays, x-rays, radio waves or gamma rays. This excites the molecules in the substance causing them to vibrate or move faster, increasing the average kinetic energy of the substance.

The First Law of Thermodynamics Now lets return to the First Law of Thermodynamics for ideal gases: Ø W is the work done on the gas. Ø This means W > 0 if the gas is compressed and W < 0 if the gas expands. Ø Q is the heat added to the gas. Ø Q > 0 if heat is added and Q < 0 if heat is removed. Ø Heat is not temperature!!!!! Ø ∆U is the change in internal energy of the gas. Ø This is where the temperature change of a gas appears. Ø ∆U = (3/2)Nk. B∆T Ø If the temperature of the gas increases ∆U > 0. If the temperature of the gas decreases ∆U < 0.

An Isobaric Process • • • In an isobaric process the pressure remains constant. In this case we can use W = -P∆V. In this case our equation for the First Law of Thermodynamics becomes: ∆U = Q - P∆V • The ideal gas law in this case reduces to:

• In an isothermal process the temperature remains constant. • This means ∆T = 0 and more importantly ∆U = 0. • In this case our equation for the First Law of Thermodynamics becomes: 0 = Q + W or Q = -W • The ideal gas law in this case reduces to: P 1 V 1 = P 2 V 2

An Adiabatic Process • In an adiabatic process there is no heat transfer. • This means Q = 0. • In this case our equation for the First Law of Thermodynamics becomes: ∆U = W • The ideal gas law in this case reduces to:

An Isovolumetric Process ØIn an isovolumetric process the volume of the gas stays constant. ØThis means W = 0. ØIn this case our equation for the First Law of Thermodynamics becomes: ∆U = Q ØThe ideal gas law in this case reduces to:
 Sssf thermodynamics
Sssf thermodynamics Isobaric process formula
Isobaric process formula First law of thermodynamics in cyclic process
First law of thermodynamics in cyclic process Joule experiment for first law of thermodynamics
Joule experiment for first law of thermodynamics Example of adiabatic process
Example of adiabatic process First law of thermodynamics compressor
First law of thermodynamics compressor First law of thermodynamics sign convention
First law of thermodynamics sign convention First law of thermodynamics control mass
First law of thermodynamics control mass Free energy
Free energy First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law Dr erukhimova
Dr erukhimova Thermodynamic
Thermodynamic Law of thermodynamics in chemistry
Law of thermodynamics in chemistry Third law of thermodynamics
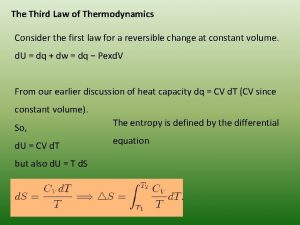
Third law of thermodynamics Work done in isentropic process
Work done in isentropic process State second law of thermodynamics
State second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics