The First Law of Thermodynamics Cyclic Processes Meeting

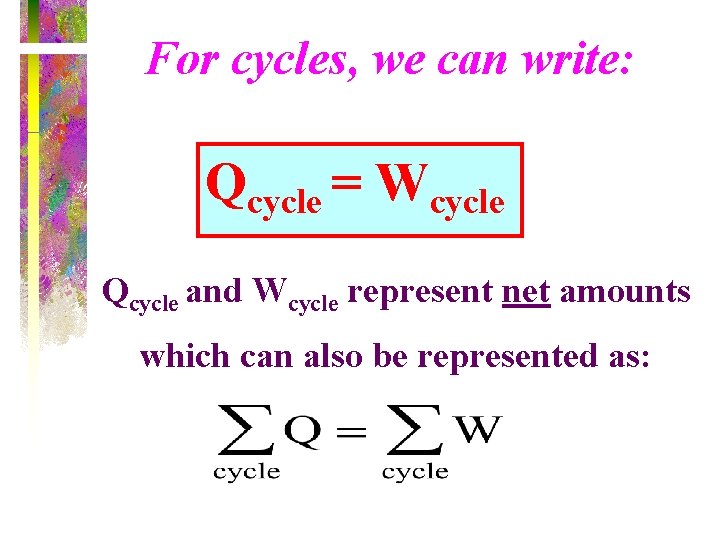


















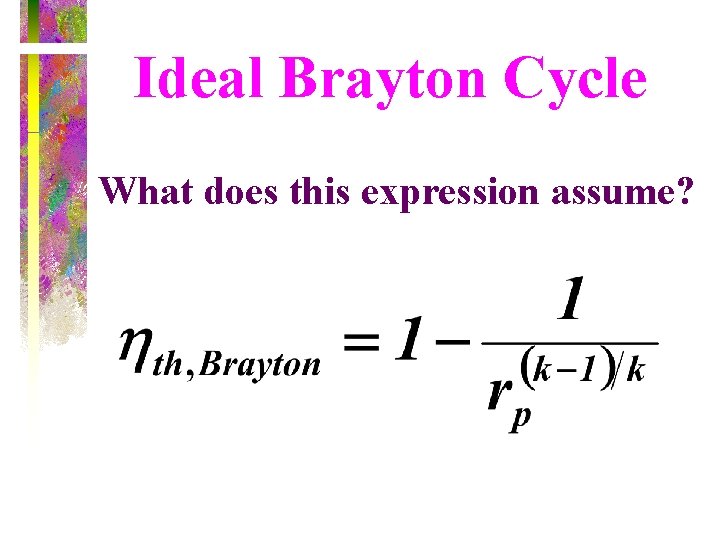



- Slides: 85

The First Law of Thermodynamics & Cyclic Processes Meeting 7 Section 4 -1

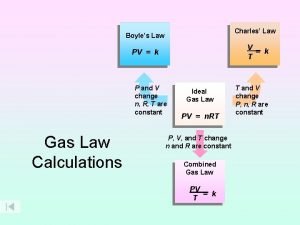
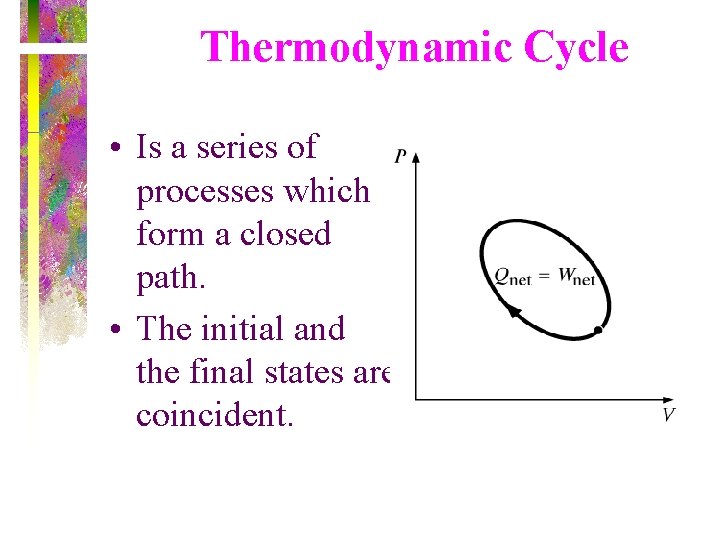
Thermodynamic Cycle • Is a series of processes which form a closed path. • The initial and the final states are coincident.

For What Thermodynamics Cycles Are For? • Thermal engines work in a cyclic process. • A Thermal engines draws heat from a hot source and rejects heat to a cold source producing work

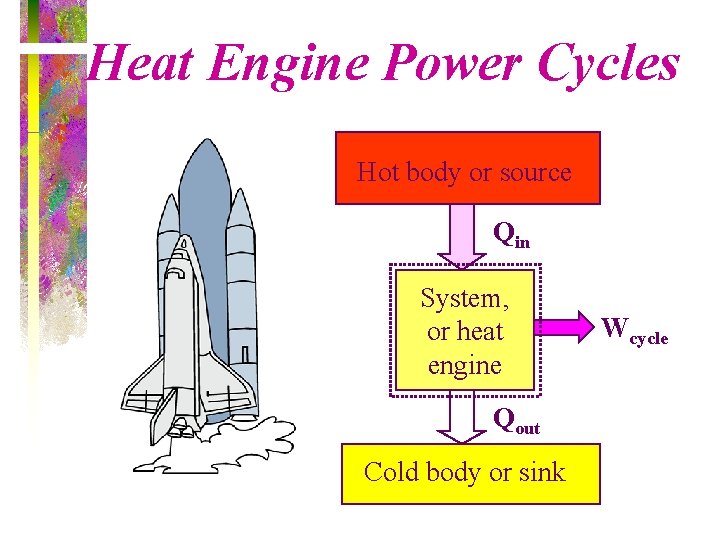
Heat Engine Power Cycles Hot body or source Qin System, or heat engine Qout Cold body or sink Wcycle

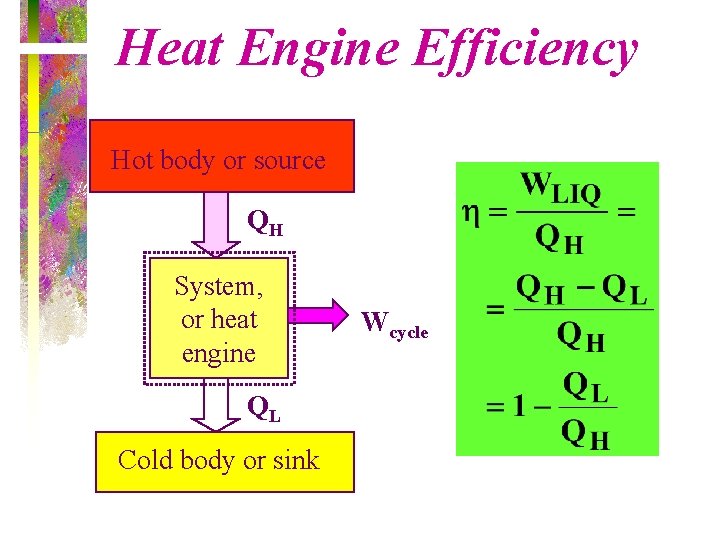
Heat Engine Efficiency Hot body or source QH System, or heat engine QL Cold body or sink Wcycle

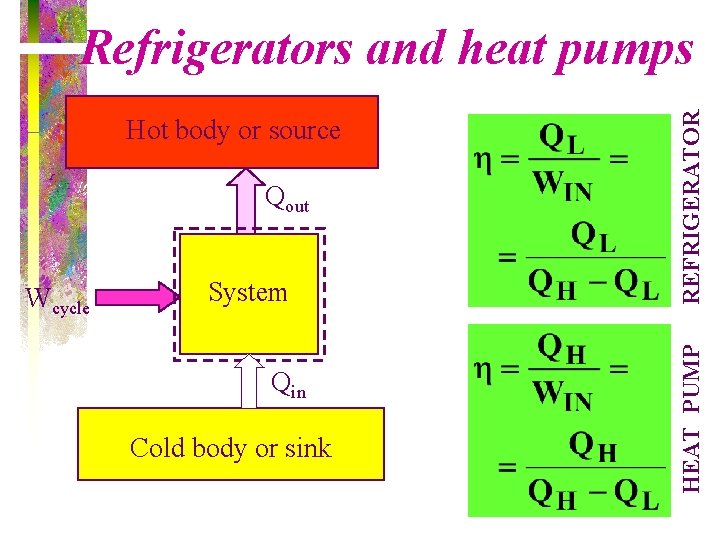
Qout Wcycle System Qin Cold body or sink HEAT PUMP Hot body or source REFRIGERATOR Refrigerators and heat pumps

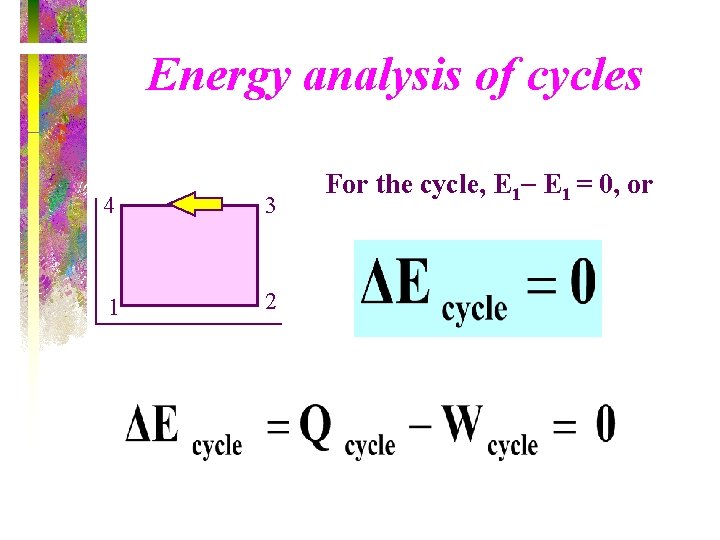
Energy analysis of cycles 4 3 1 2 For the cycle, E 1 = 0, or
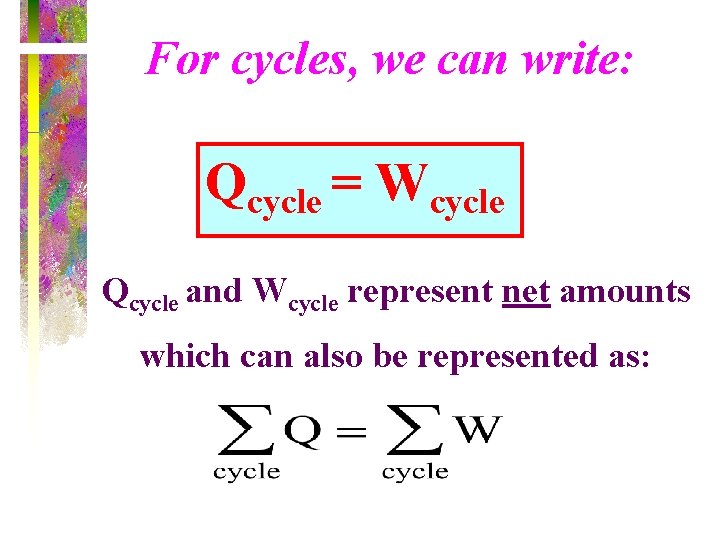
For cycles, we can write: Qcycle = Wcycle Qcycle and Wcycle represent net amounts which can also be represented as:

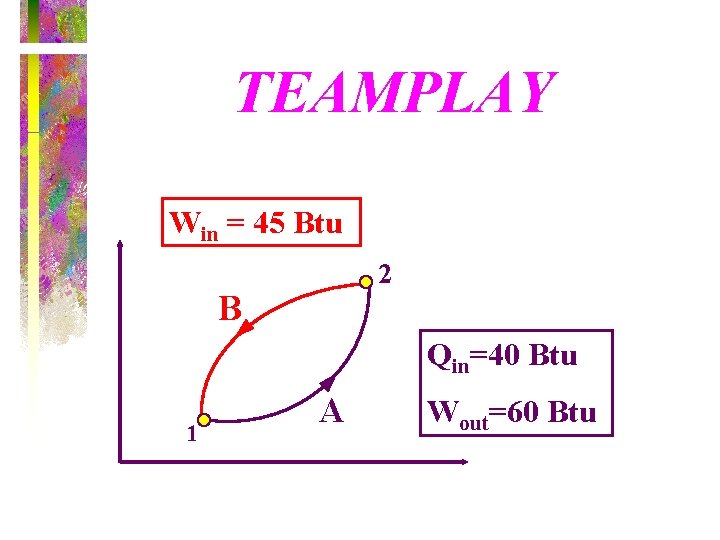
TEAMPLAY • A closed system undergoes a cycle consisting of two processes. During the first process, 40 Btu of heat is transferred to the system while the system does 60 Btu of work. During the second process, 45 Btu of work is done on the system. (a) Determine the heat transfer during the second process. (b) Calculate the net work and net heat transfer of the cycle.

TEAMPLAY Win = 45 Btu 2 B Qin=40 Btu 1 A Wout=60 Btu


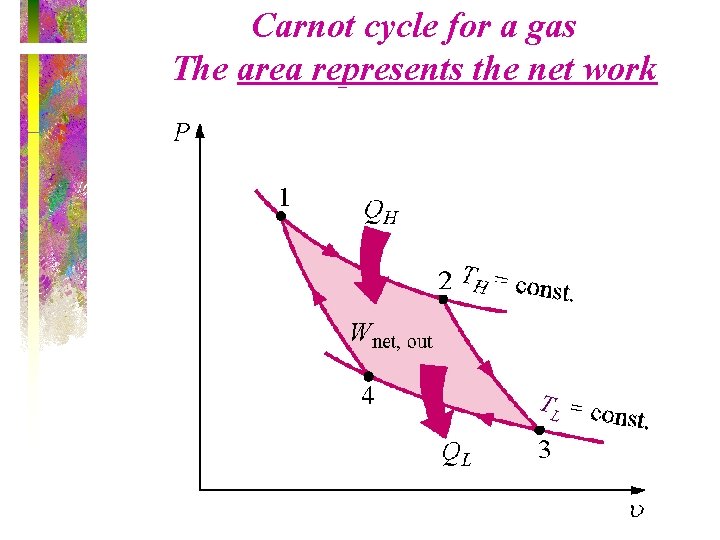
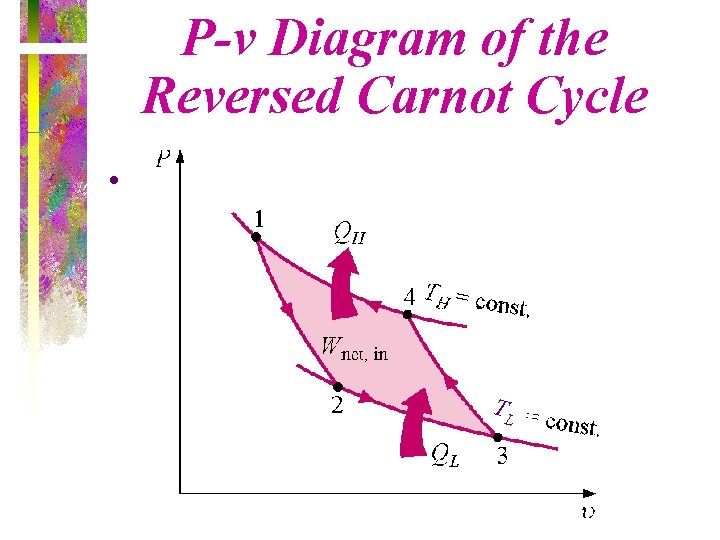
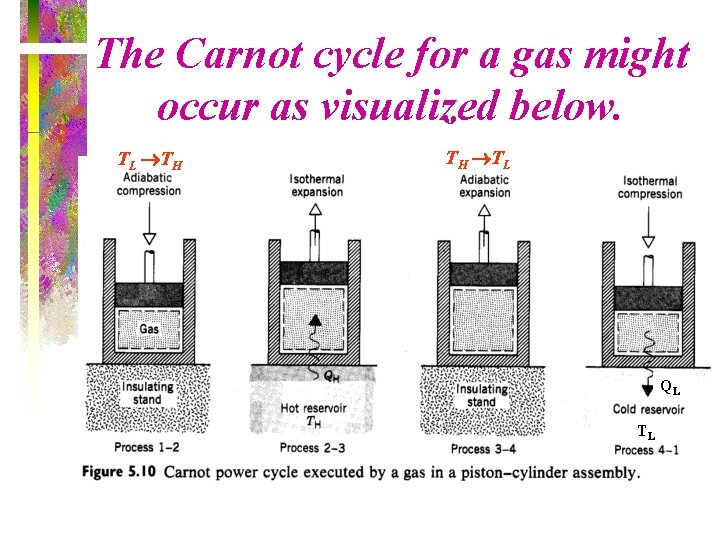
Carnot Cycle • The Carnot cycle is a reversible cycle that is composed of four internally reversible processes. – Two isothermal processes – Two adiabatic processes

Carnot cycle for a gas The area represents the net work TL

P-v Diagram of the Reversed Carnot Cycle • TL

The Carnot cycle for a gas might occur as visualized below. TL TH TH TL QL TL

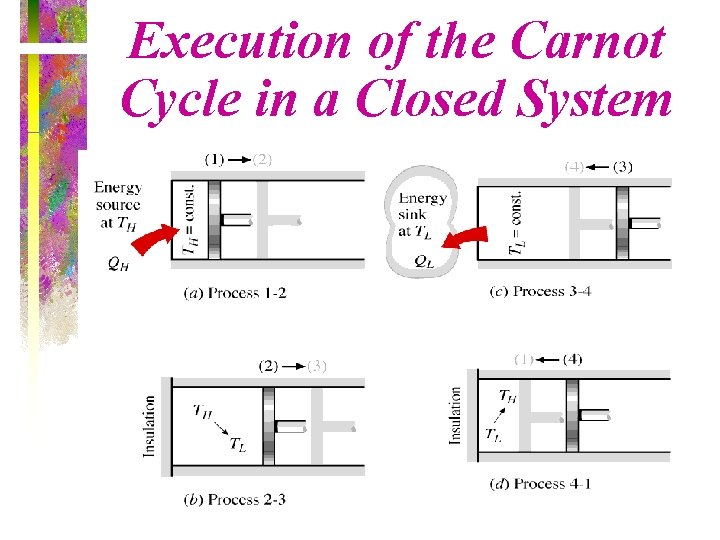
Execution of the Carnot Cycle in a Closed System • (Fig. 5 -43)

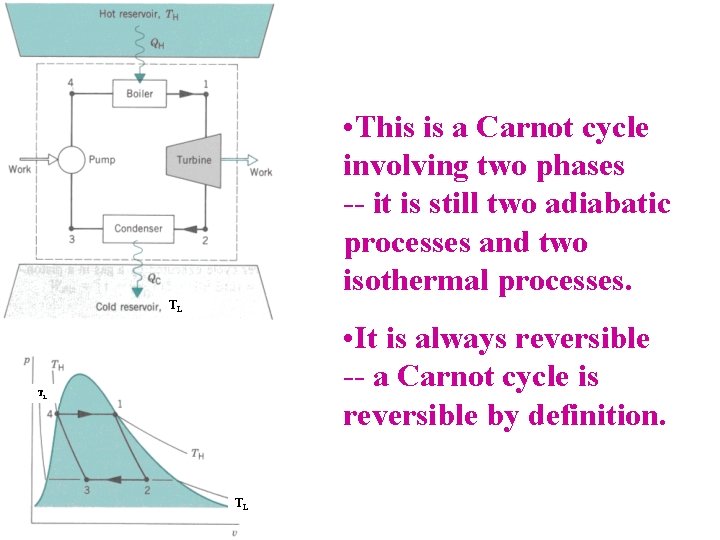
• This is a Carnot cycle involving two phases -- it is still two adiabatic processes and two isothermal processes. TL • It is always reversible -- a Carnot cycle is reversible by definition. TL TL

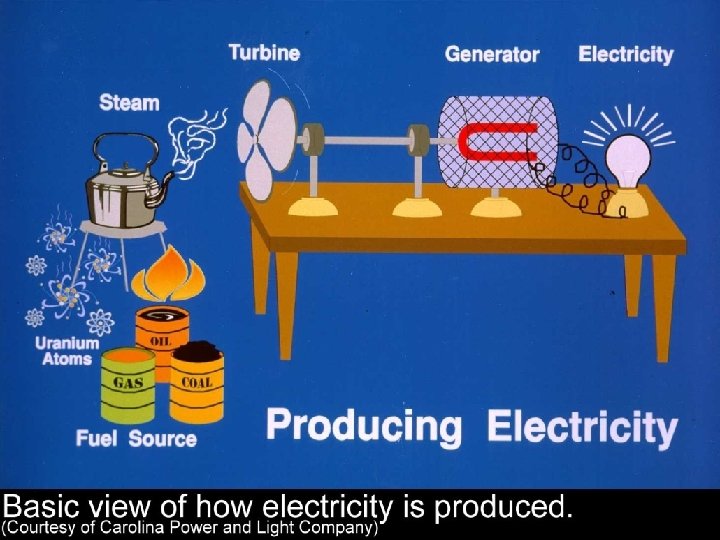
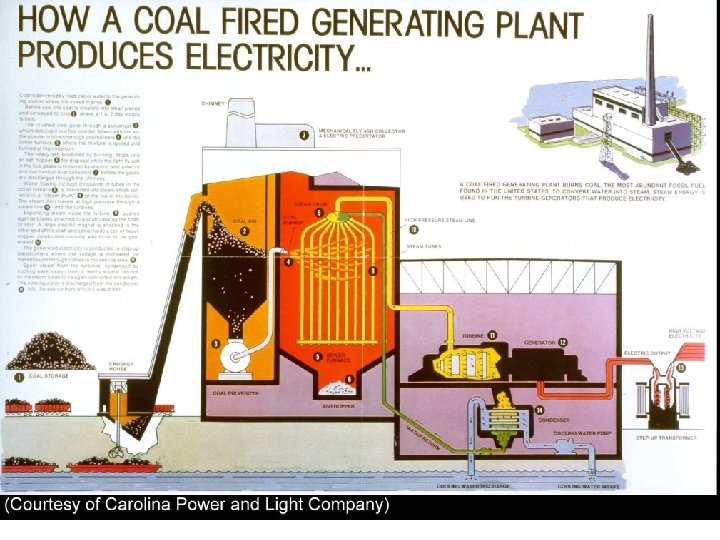
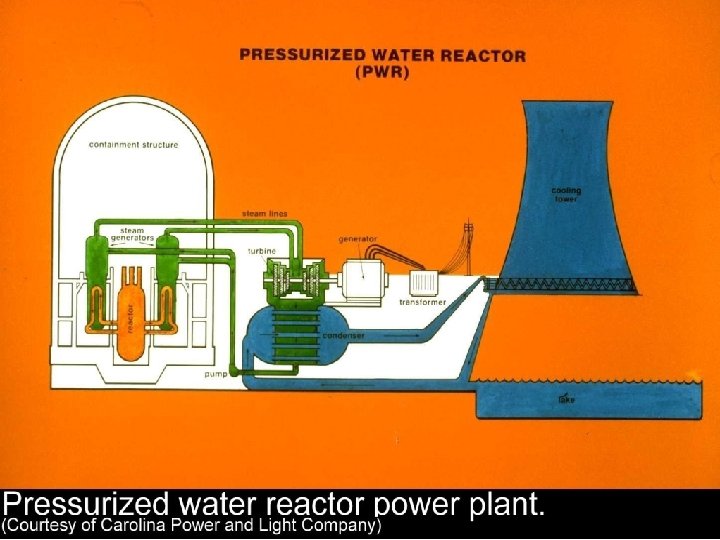
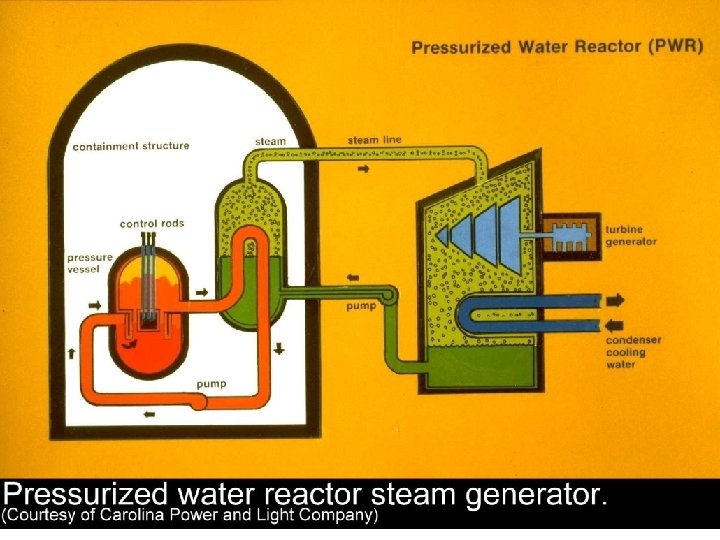
Vapor Power Cycles • We’ll look specifically at the Rankine cycle, which is a vapor power cycle. • It is the primary electrical producing cycle in the world. • The cycle can use a variety of fuels.










Question …. . How much does it cost to operate a gas fired 1000 MW(output) power plant with a 35% efficiency for 24 hours/day for a full year if fuel cost are $2. 00 per 106 Btu? $467, 952. 27/day $170, 801, 979/year

Question …. If you could improve the efficiency of a 1000 MW power plant from 35% to 36%, what would be a reasonable charge for your services? Let’s assume $2. 00 per million BTUs fuel charge and 24 hr/day operation. $12, 998. 67/day $4, 744, 499/year

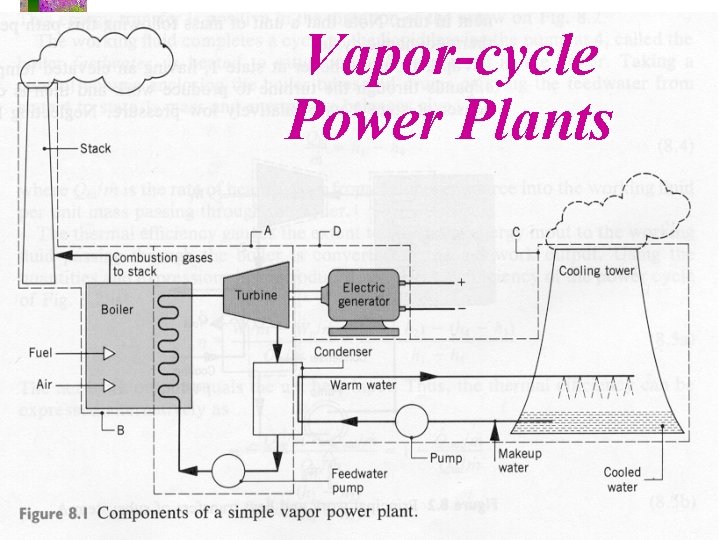
Vapor-cycle Power Plants

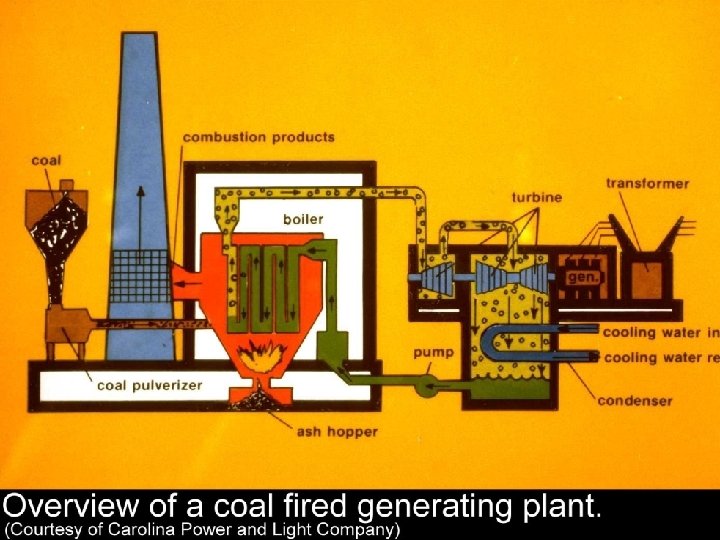
We’ll simplify the power plant

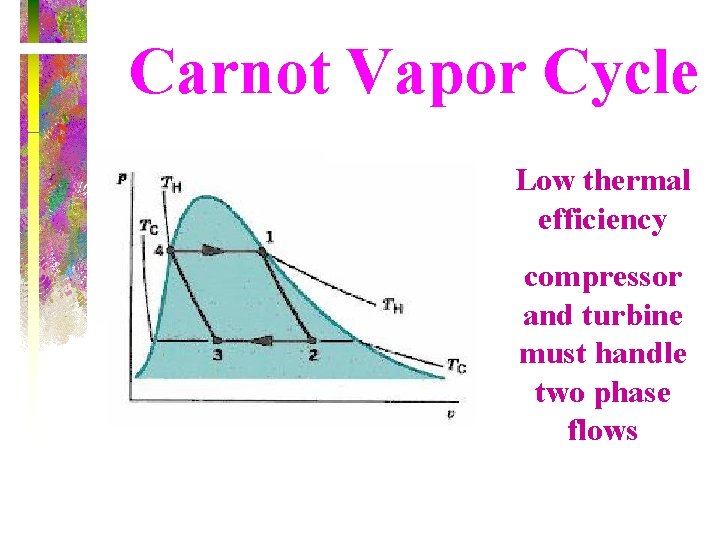
Carnot Vapor Cycle Low thermal efficiency compressor and turbine must handle two phase flows

Carnot Vapor Cycle • The Carnot cycle is not a suitable model for vapor power cycles because it cannot be approximated in practice.

Rankine Cycle • The model cycle for vapor power cycles is the Rankine cycle which is composed of four internally reversible processes: constantpressure heat addition in a boiler, isentropic expansion in a turbine, constant-pressure heat rejection in a condenser, and isentropic compression in a pump. Steam leaves the condenser as a saturated liquid at the condenser pressure.

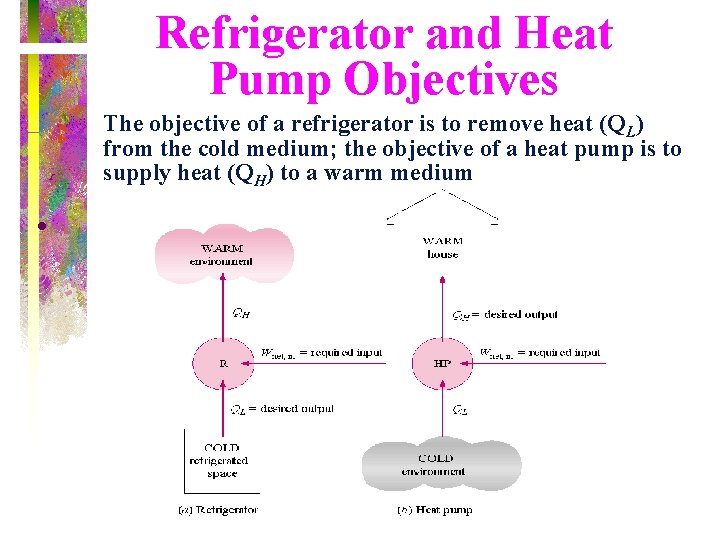
Refrigerator and Heat Pump Objectives The objective of a refrigerator is to remove heat (QL) from the cold medium; the objective of a heat pump is to supply heat (QH) to a warm medium •

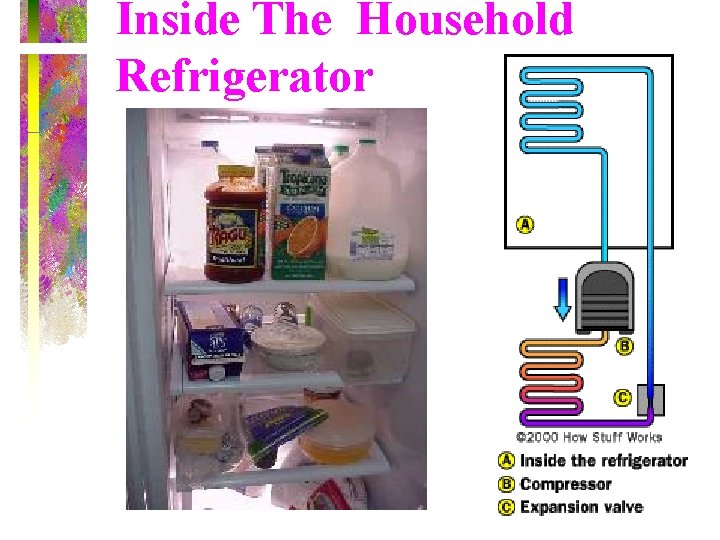
Inside The Household Refrigerator

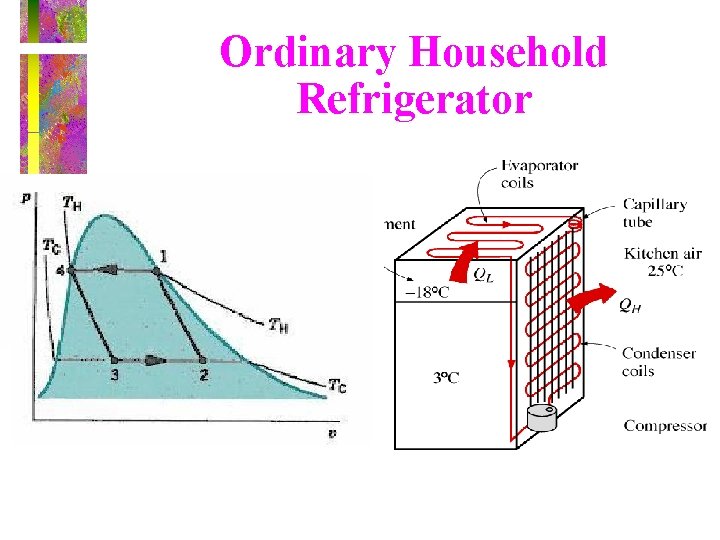
Ordinary Household Refrigerator

Gas Power Cycle • A cycle during which a net amount of work is produced is called a power cycle, and a power cycle during which the working fluid remains a gas throughout is called a gas power cycle.


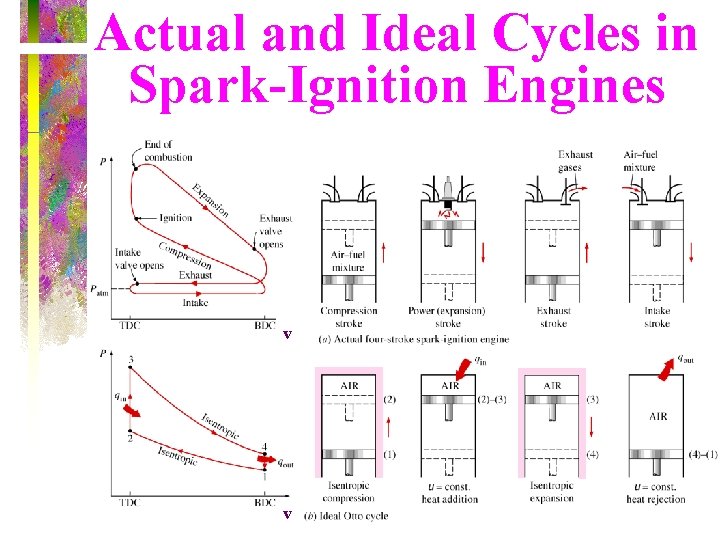
Actual and Ideal Cycles in Spark-Ignition Engines v v

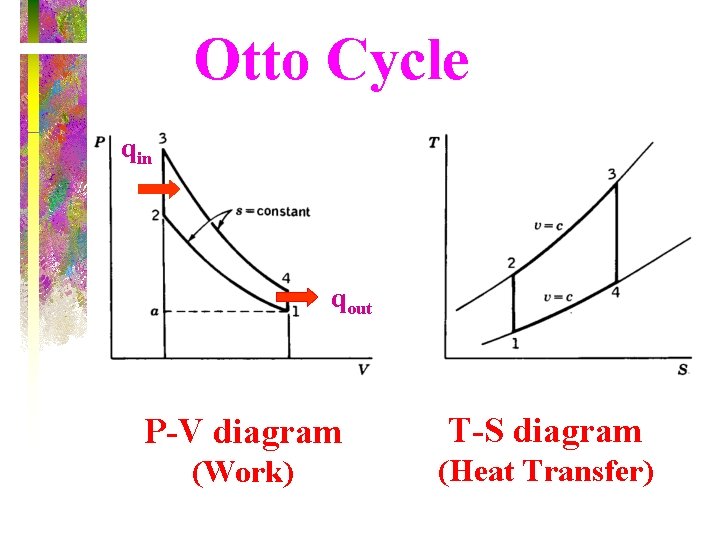
Otto Cycle qin qout P-V diagram T-S diagram (Work) (Heat Transfer)

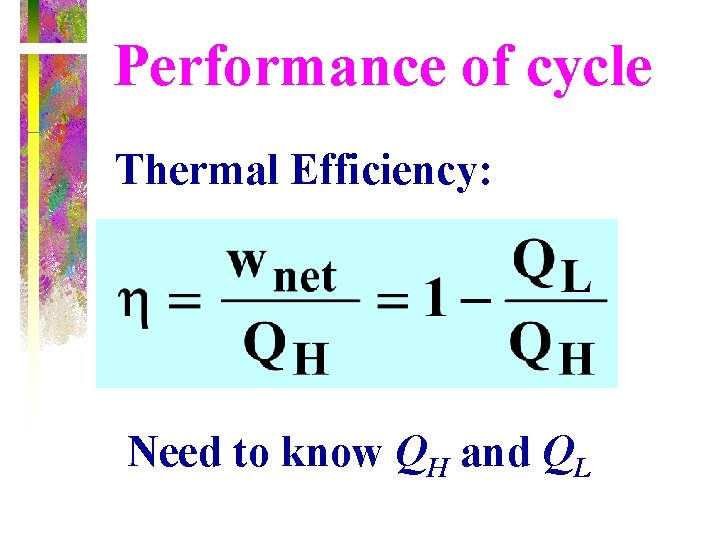
Performance of cycle Thermal Efficiency: Need to know QH and QL

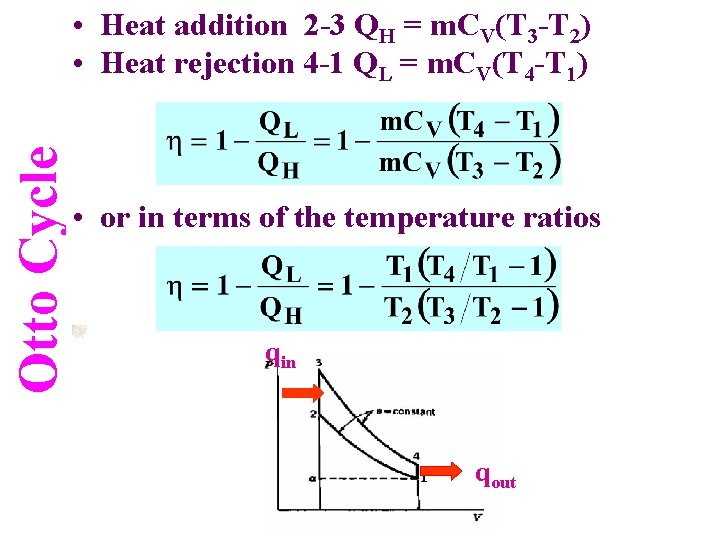
Otto Cycle • Heat addition 2 -3 QH = m. CV(T 3 -T 2) • Heat rejection 4 -1 QL = m. CV(T 4 -T 1) • or in terms of the temperature ratios qin qout

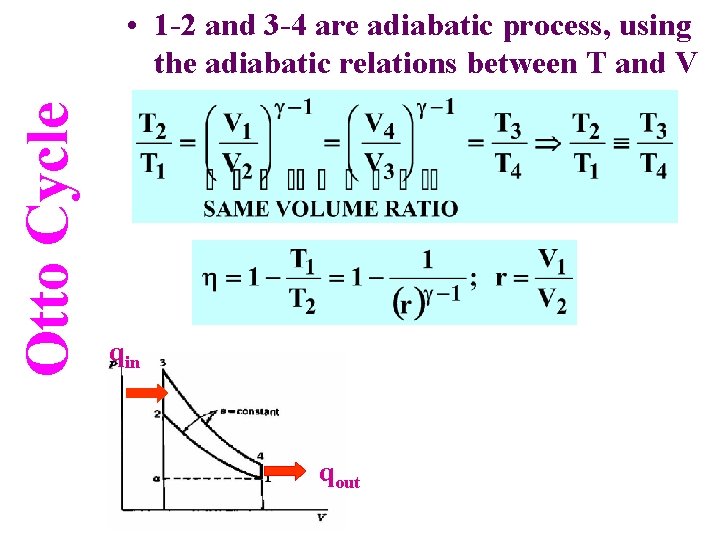
Otto Cycle • 1 -2 and 3 -4 are adiabatic process, using the adiabatic relations between T and V qin qout

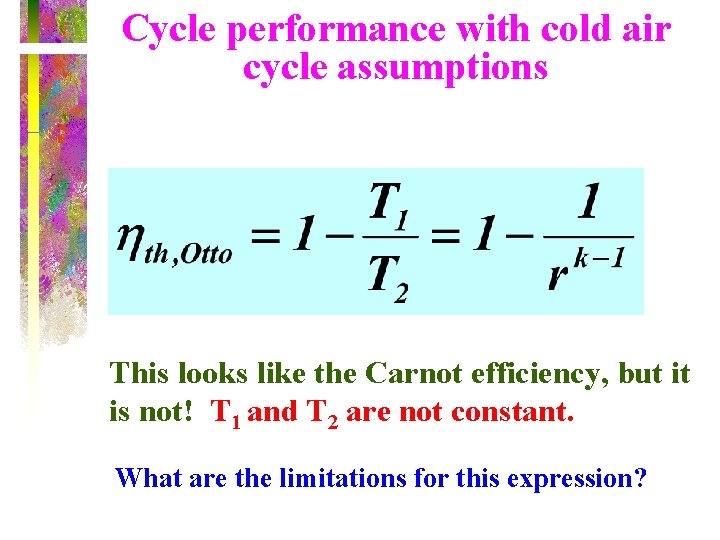
Cycle performance with cold air cycle assumptions This looks like the Carnot efficiency, but it is not! T 1 and T 2 are not constant. What are the limitations for this expression?

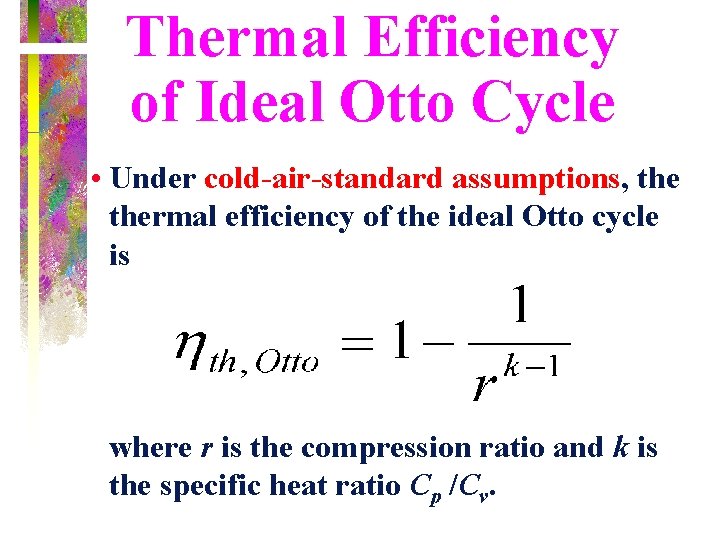
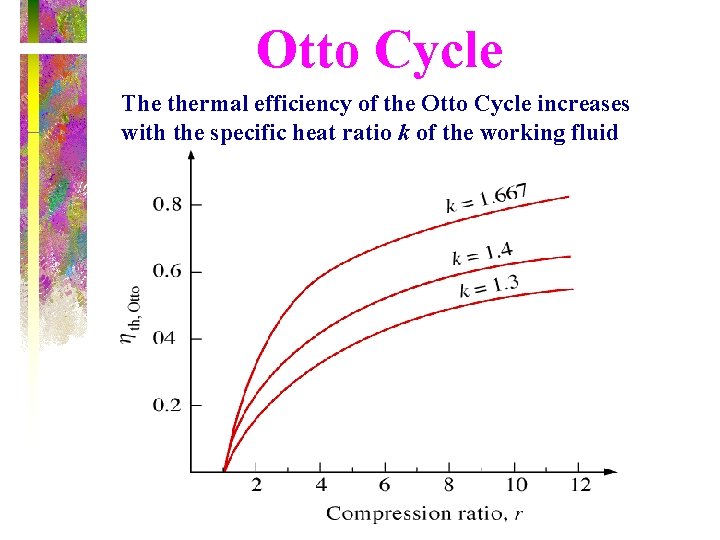
Thermal Efficiency of Ideal Otto Cycle • Under cold-air-standard assumptions, thermal efficiency of the ideal Otto cycle is where r is the compression ratio and k is the specific heat ratio Cp /Cv.

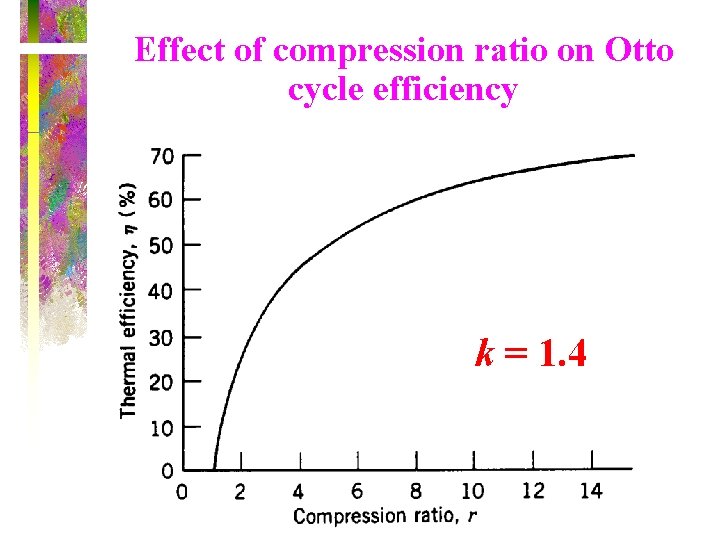
Effect of compression ratio on Otto cycle efficiency k = 1. 4

Otto Cycle The thermal efficiency of the Otto Cycle increases with the specific heat ratio k of the working fluid

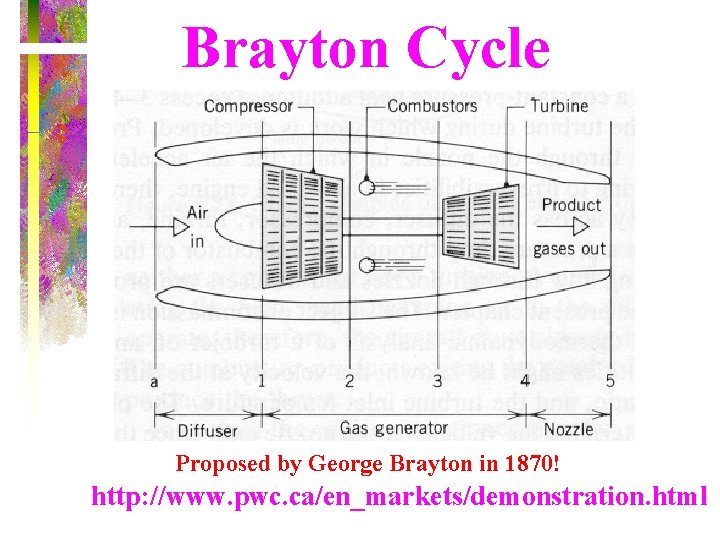
Brayton Cycle • This is another air standard cycle and it models modern turbojet engines.


Brayton Cycle Proposed by George Brayton in 1870! http: //www. pwc. ca/en_markets/demonstration. html

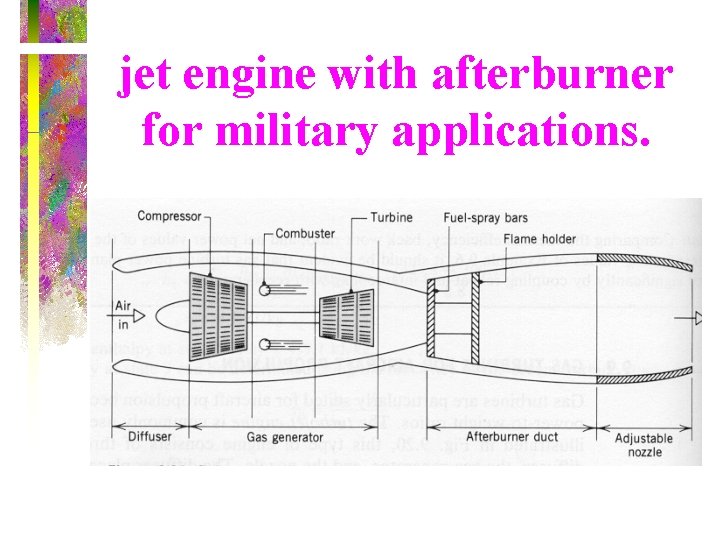
jet engine with afterburner for military applications.

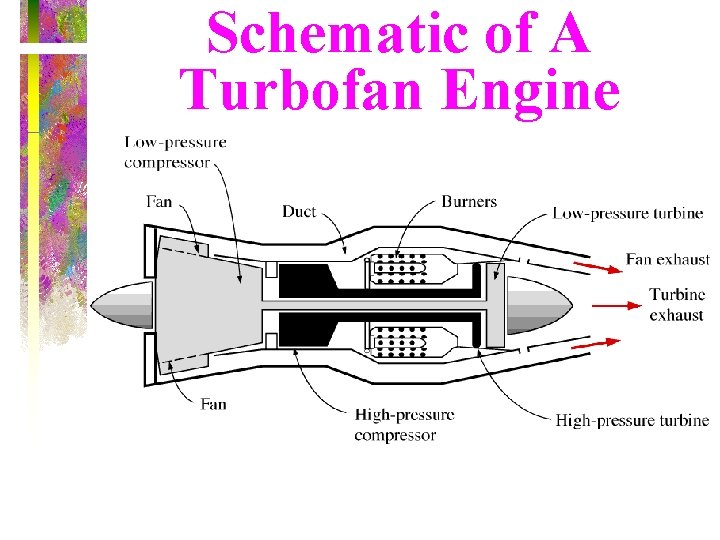
Schematic of A Turbofan Engine

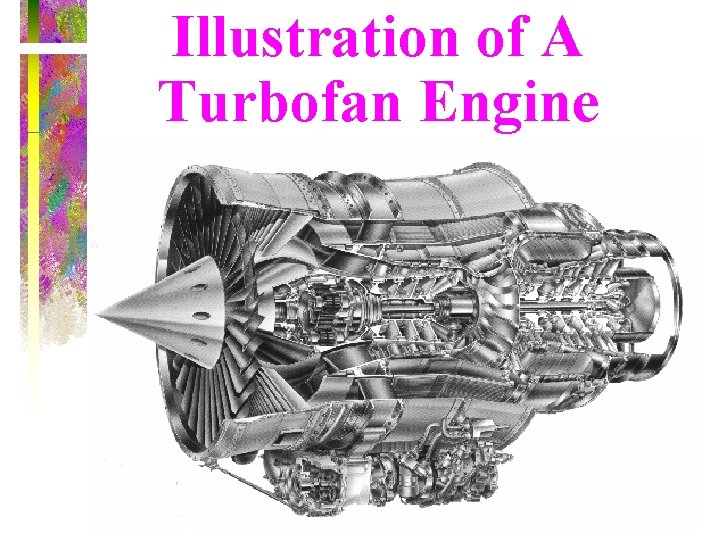
Illustration of A Turbofan Engine

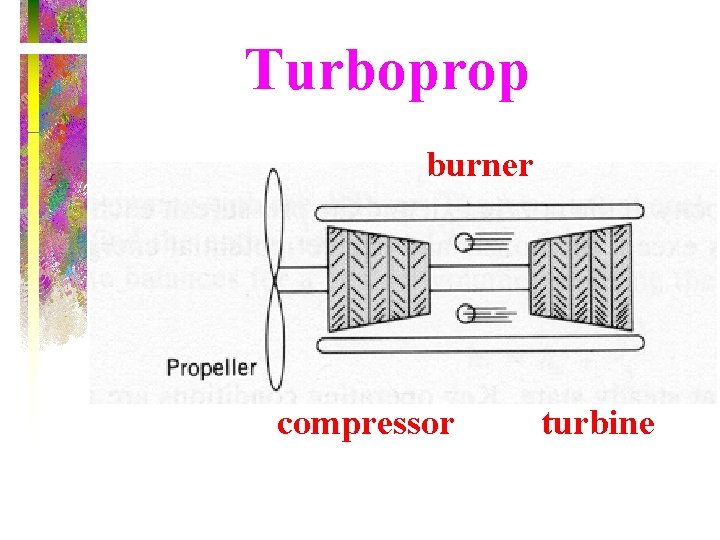
Turboprop burner compressor turbine

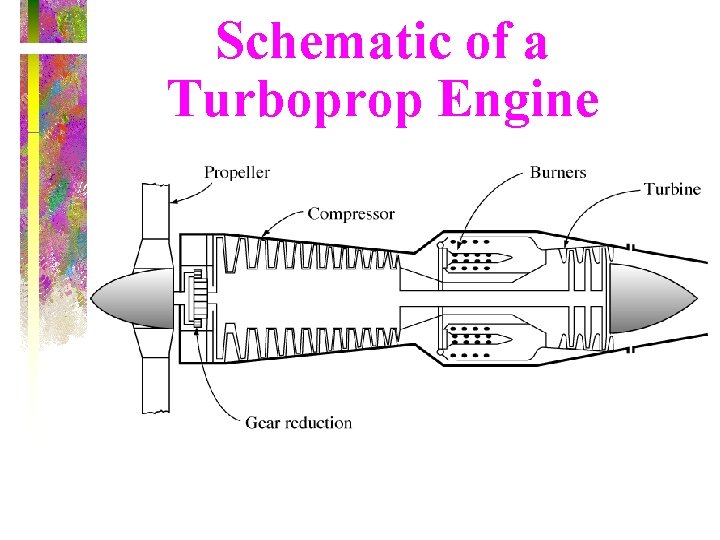
Schematic of a Turboprop Engine

Other applications of Brayton cycle • Power generation - use gas turbines to generate electricity…very efficient • Marine applications in large ships • Automobile racing - late 1960 s Indy 500 STP sponsored cars

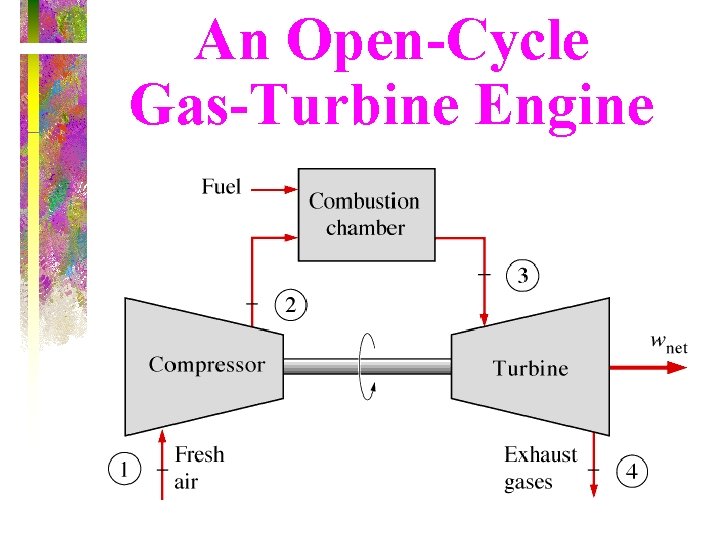
An Open-Cycle Gas-Turbine Engine

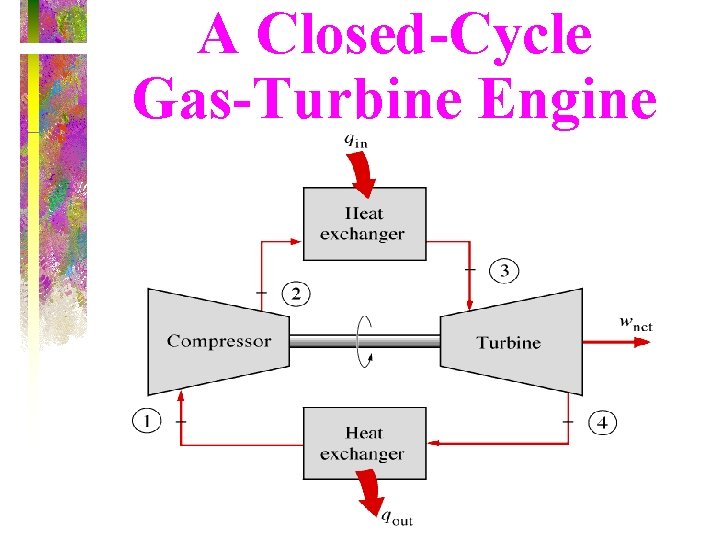
A Closed-Cycle Gas-Turbine Engine

Brayton Cycle • The ideal cycle for modern gasturbine engines is the Brayton cycle, which is made up of four internally reversible processes: isentropic compression, constant pressure heat addition, isentropic expansion, and constant pressure heat rejection.

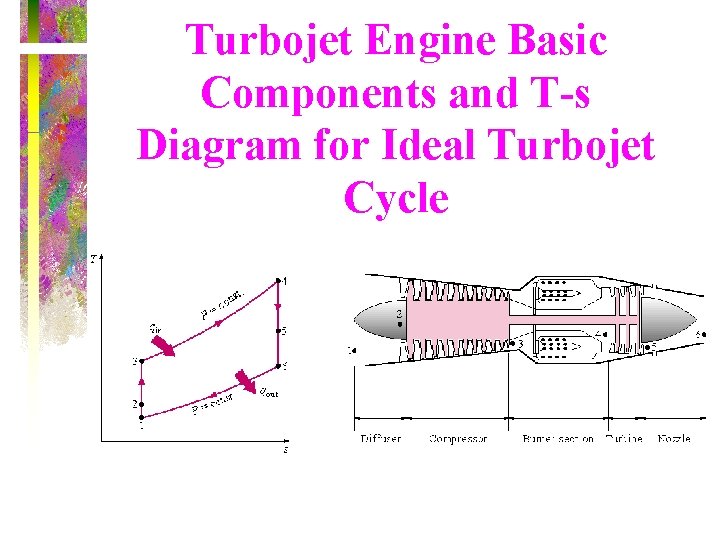
Turbojet Engine Basic Components and T-s Diagram for Ideal Turbojet Cycle

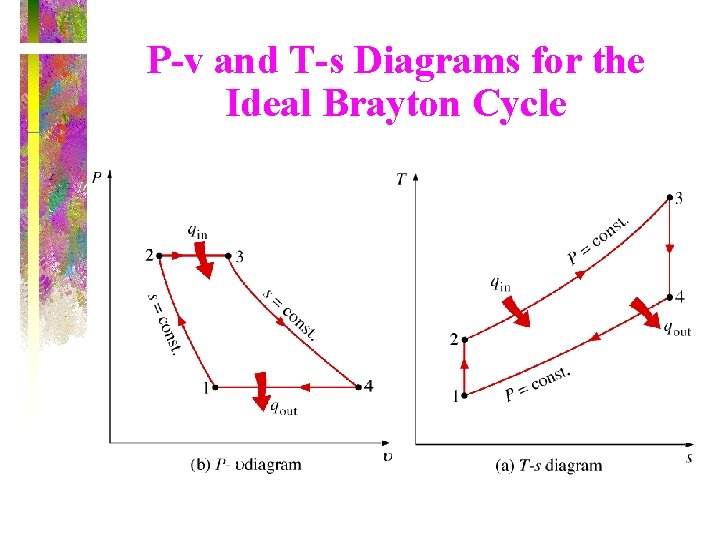
P-v and T-s Diagrams for the Ideal Brayton Cycle

Brayton Cycle • 1 to 2 --isentropic compression in the compressor • 2 to 3 --constant pressure heat addition (replaces combustion process) • 3 to 4 --isentropic expansion in the turbine • 4 to 1 --constant pressure heat rejection to return air to original state

Brayton Cycle • Because the Brayton cycle operates between two constant pressure lines, or isobars, the pressure ratio is important. • The pressure ratio is not a compression ratio.

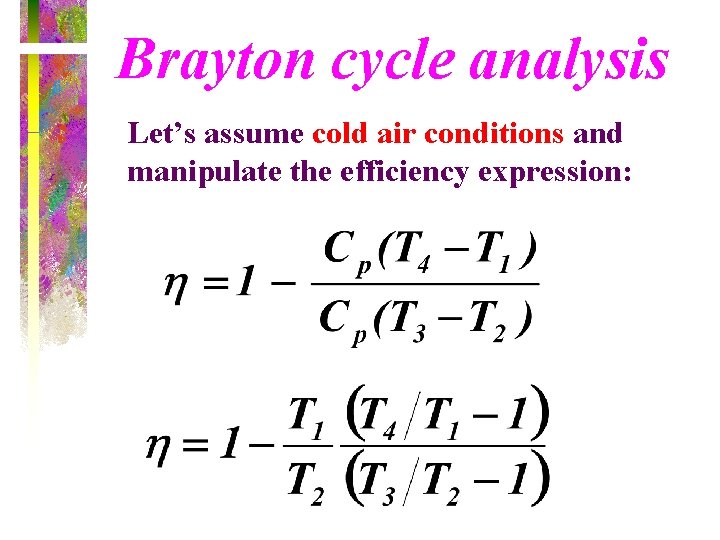
Brayton cycle analysis Let’s assume cold air conditions and manipulate the efficiency expression:

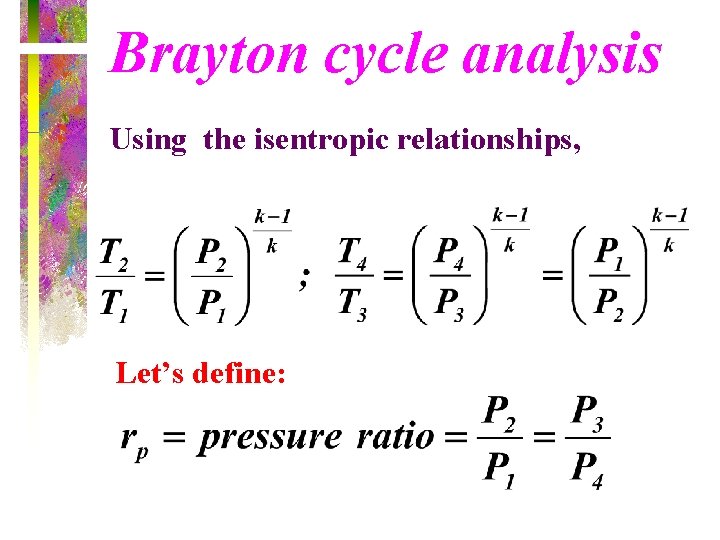
Brayton cycle analysis Using the isentropic relationships, Let’s define:

Brayton Cycle • Because the Brayton cycle operates between two constant pressure lines, or isobars, the pressure ratio is important. • The pressure ratio is just that--a pressure ratio. • A compression ratio is a volume ratio (refer to the Otto Cycle).

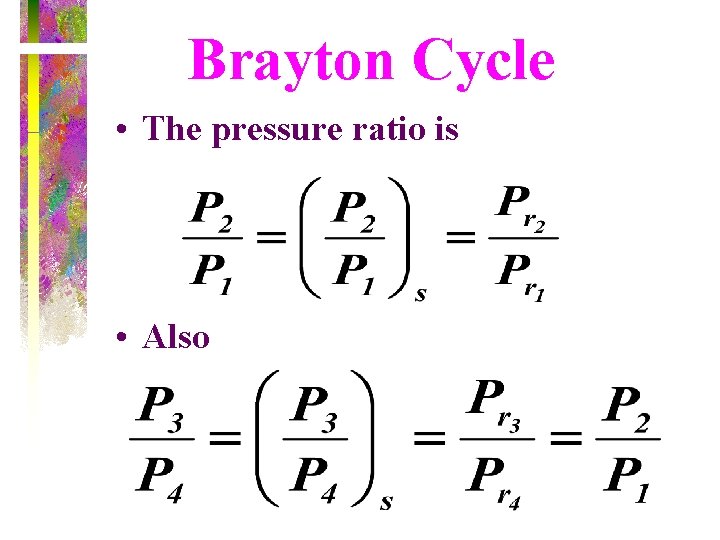
Brayton Cycle • The pressure ratio is • Also

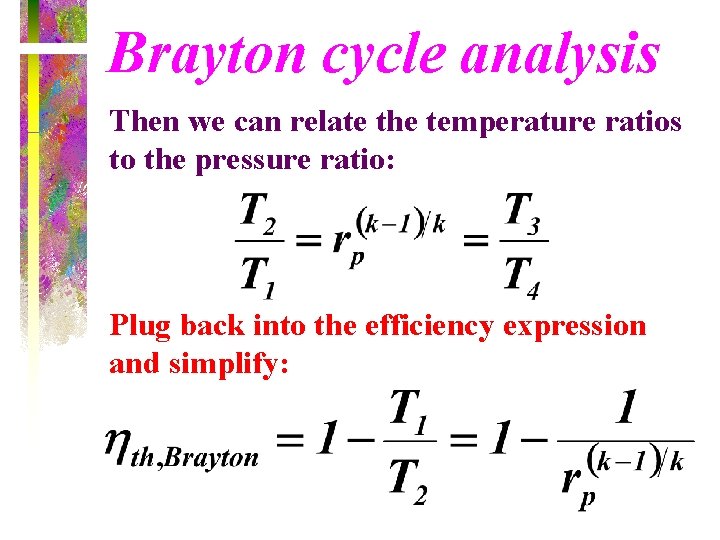
Brayton cycle analysis Then we can relate the temperature ratios to the pressure ratio: Plug back into the efficiency expression and simplify:
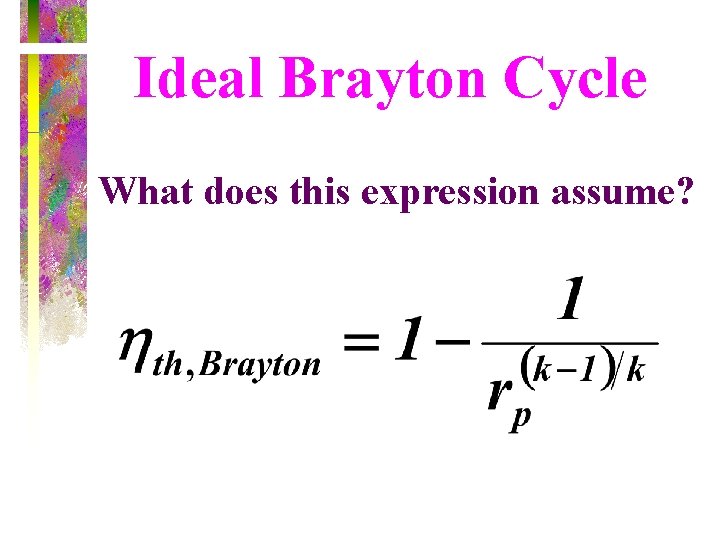
Ideal Brayton Cycle What does this expression assume?

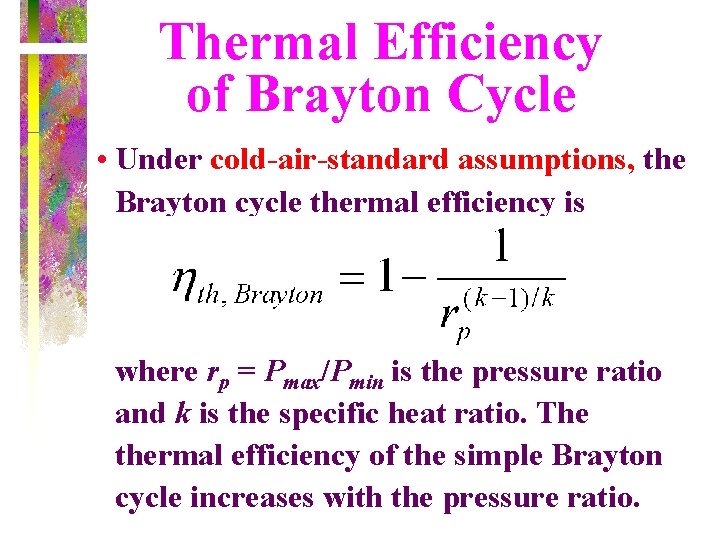
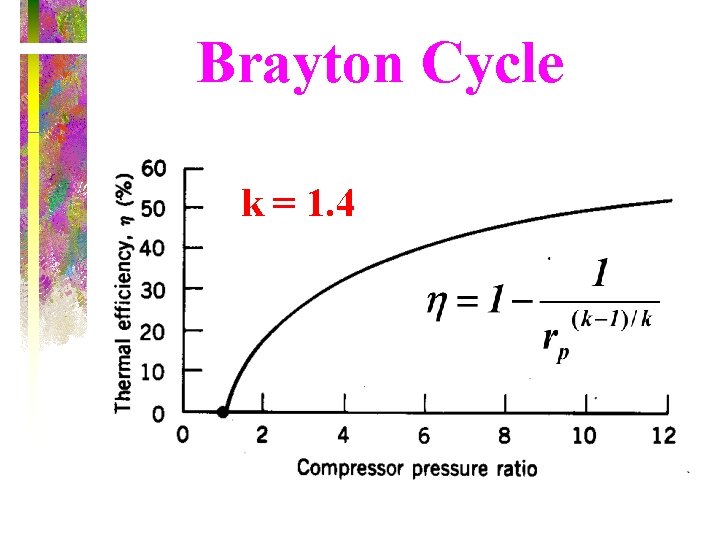
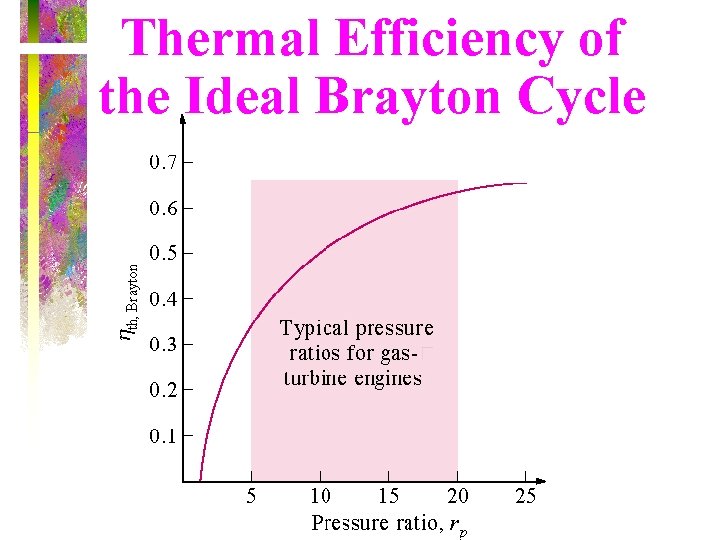
Thermal Efficiency of Brayton Cycle • Under cold-air-standard assumptions, the Brayton cycle thermal efficiency is where rp = Pmax/Pmin is the pressure ratio and k is the specific heat ratio. The thermal efficiency of the simple Brayton cycle increases with the pressure ratio.

Brayton Cycle k = 1. 4

Thermal Efficiency of the Ideal Brayton Cycle

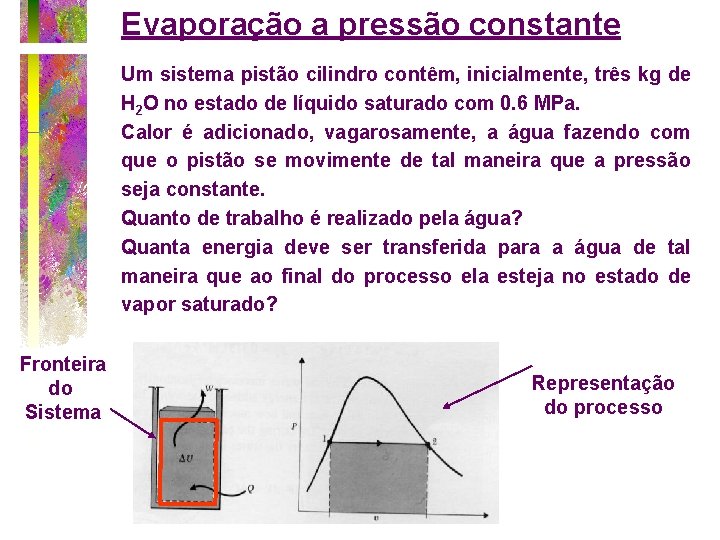
Evaporação a pressão constante Um sistema pistão cilindro contêm, inicialmente, três kg de H 2 O no estado de líquido saturado com 0. 6 MPa. Calor é adicionado, vagarosamente, a água fazendo com que o pistão se movimente de tal maneira que a pressão seja constante. Quanto de trabalho é realizado pela água? Quanta energia deve ser transferida para a água de tal maneira que ao final do processo ela esteja no estado de vapor saturado? Fronteira do Sistema Representação do processo

processo a pressão const. Primeira Lei: 1 Q 2 – 1 W 2 = U 2 – U 1 mas o trabalho 1 W 2 = Patm*(V 2 -V 1), Logo 1 Q 2 = (P 2 V 2+U 2)-(P 1 V 1+U 1) = H 2 -H 1 Onde h 2 é a entalpia do vapor saturado e h 1 é a entalpia do líquido. Na tabela 1 -2 termodinâmico para 0. 6 MPa, tem-se que h 2 = 2756, 8 KJ/kg e h 1 = 670, 56 KJ/kg. Considerando 3 kg de H 2 O, então o calor transferido será de 3*(2756 -670) = 6259 KJ.

Resfriamento com Gelo Seco 0. 5 kg de gelo seco (CO 2) a 1 atm é colocado em cima de uma fatia de picanha. O gelo seco sublima a pressão constante devido ao fluxo de calor transferido pela picanha. Ao final do processo todo CO 2 está no estado de vapor (foi completamente sublimado). Determine a temperatura do CO 2 e quanto de calor ele recebeu da picanha. Fronteira do Sistema Representação do processo

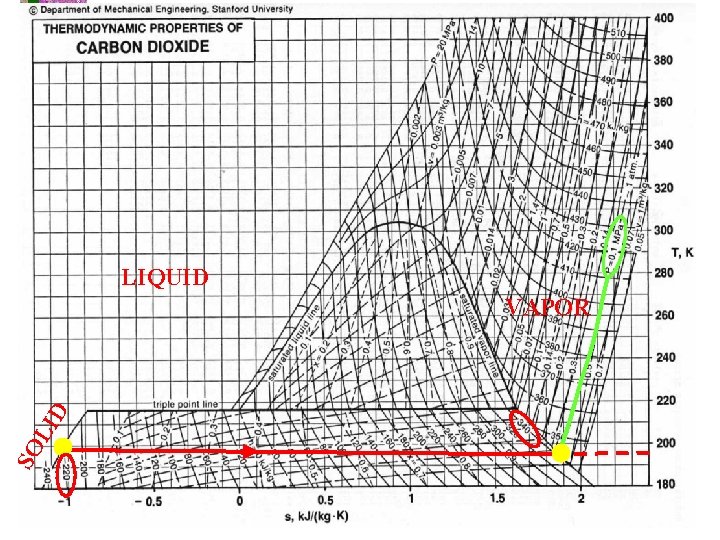
Resfriamento com Gelo Seco – processo a pressão const. Primeira Lei: 1 Q 2 – 1 W 2 = U 2 – U 1 mas o trabalho 1 W 2 = Patm*(V 2 -V 1), Logo 1 Q 2 = (P 2 V 2+U 2)-(P 1 V 1+U 1) = H 2 -H 1 Onde h 2 é a entalpia do vapor saturado e h 1 é a entalpia do sólido. No diagrama termodinâmico para Patm, tem-se que h 2 = 340 KJ/kg e h 1 = -220 KJ/kg. Considerando 0. 5 kg de CO 2, então o calor transferido será de 280 KJ. A temperatura de saturação do CO 2 será de 175 K (-98 o. C)

LIQUID SO LI D VAPOR

Solução de Exercícios Cap 4

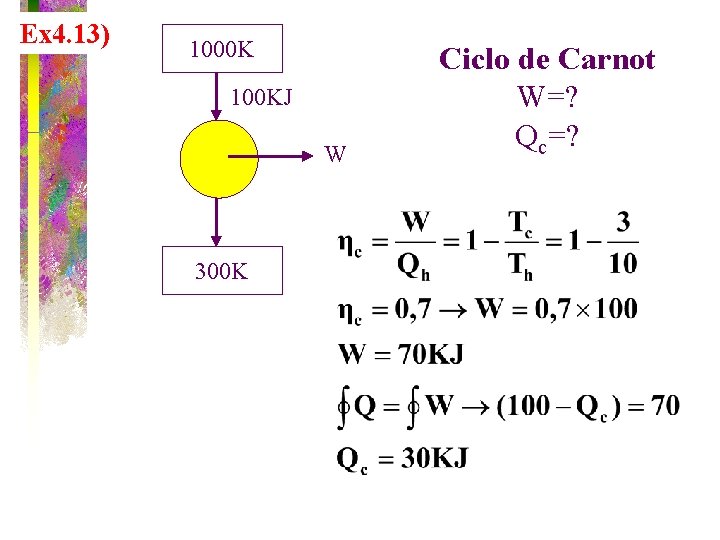
Ex 4. 13) 1000 K 100 KJ W 300 K Ciclo de Carnot W=? Qc=?

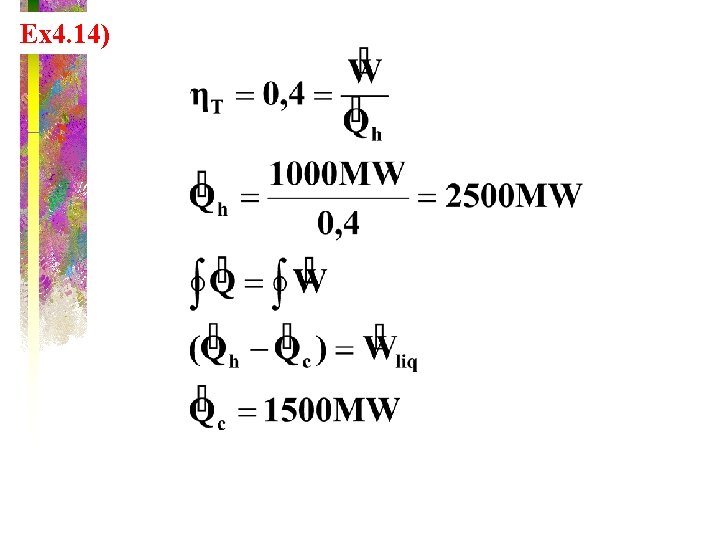
Ex 4. 14)

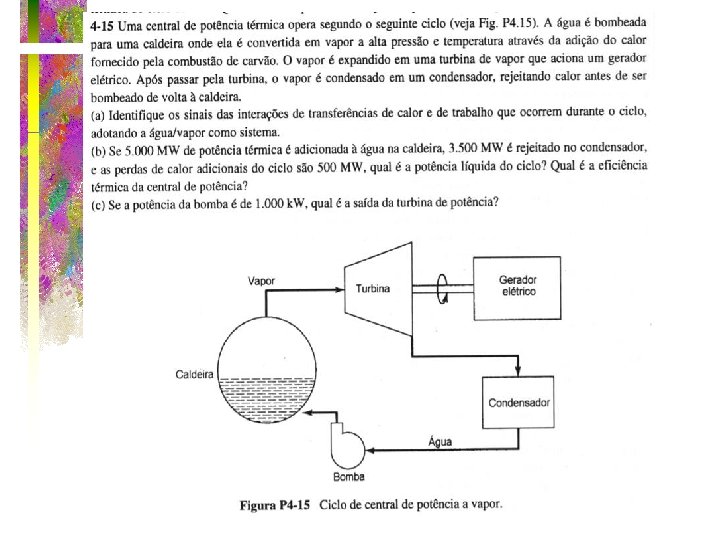
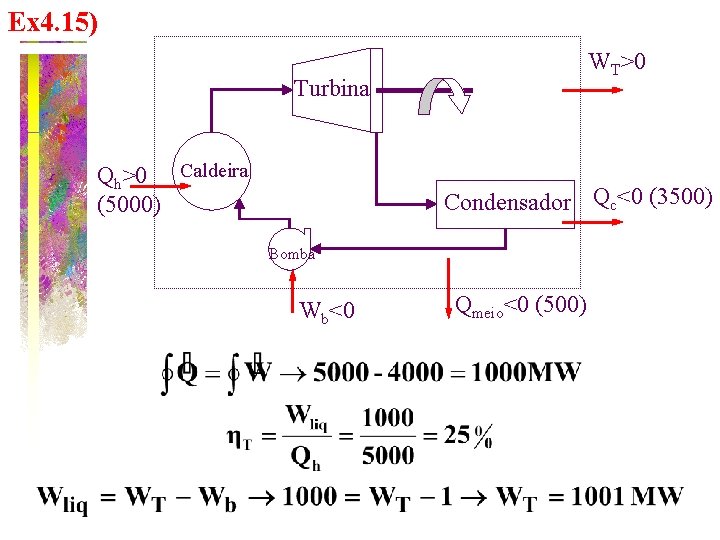
Ex 4. 15) WT>0 Turbina Qh>0 Caldeira (5000) Condensador Qc<0 (3500) Bomba Wb<0 Qmeio<0 (500)

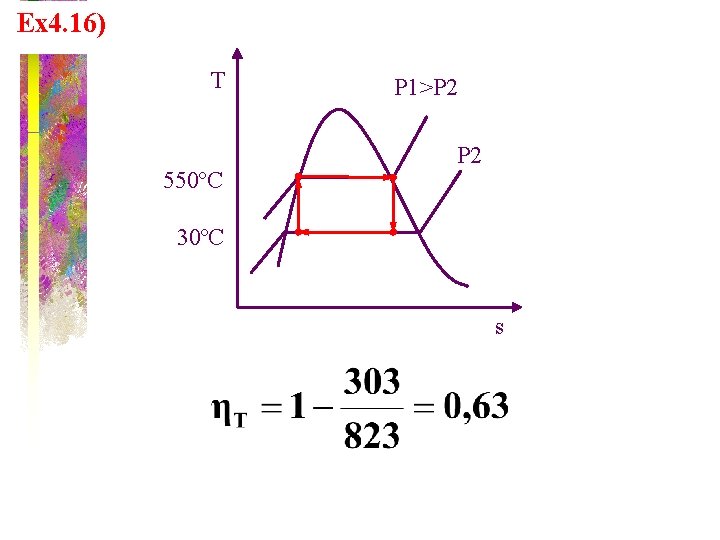
Ex 4. 16) T 550ºC P 1>P 2 30ºC s

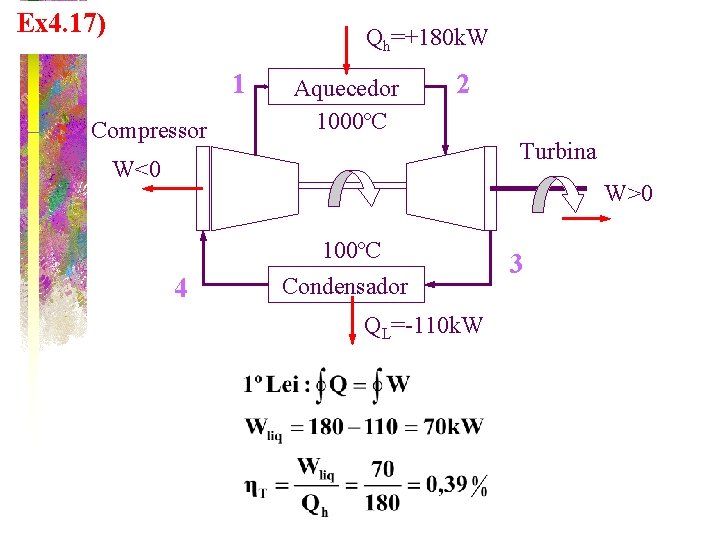
Ex 4. 17) Qh=+180 k. W 1 Compressor Aquecedor 1000ºC 2 Turbina W<0 W>0 4 100ºC Condensador QL=-110 k. W 3

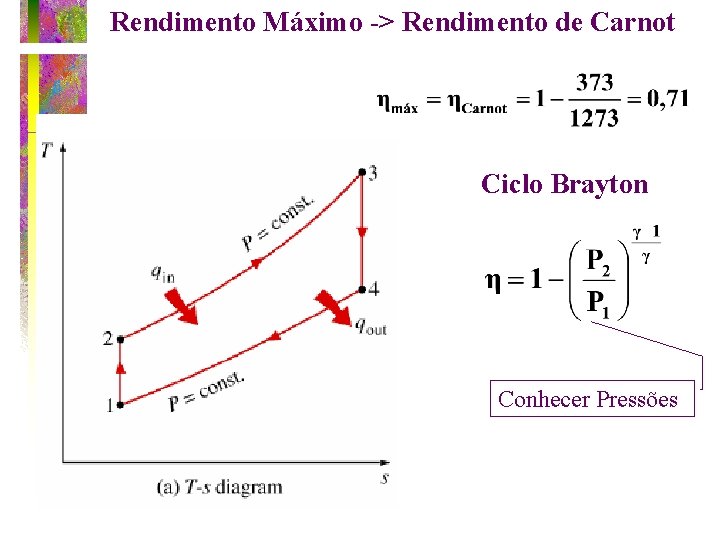
Rendimento Máximo -> Rendimento de Carnot Ciclo Brayton Conhecer Pressões
 First law of thermodynamics cyclic process
First law of thermodynamics cyclic process Energy balance equation thermodynamics open system
Energy balance equation thermodynamics open system First law of thermodynamics
First law of thermodynamics Joule experiment for first law of thermodynamics
Joule experiment for first law of thermodynamics Example of adiabatic process
Example of adiabatic process In a flow process the work transfer may be of which type
In a flow process the work transfer may be of which type First law of thermodynamics sign convention
First law of thermodynamics sign convention Enthalpy vs internal energy
Enthalpy vs internal energy Gibbs free energy
Gibbs free energy Thermodynamics of ideal gases
Thermodynamics of ideal gases Newton's first law and second law and third law
Newton's first law and second law and third law Si unit of newton's first law
Si unit of newton's first law Reversible and irreversible processes in thermodynamics
Reversible and irreversible processes in thermodynamics Concurrent in os
Concurrent in os Dr erukhimova
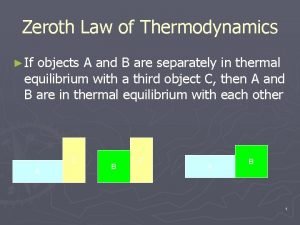
Dr erukhimova Oth law of thermodynamics
Oth law of thermodynamics Newton's third law of thermodynamics
Newton's third law of thermodynamics Zeroth law of thermodynamics examples
Zeroth law of thermodynamics examples Kelvin planck statement
Kelvin planck statement Zeroth law of thermodynamics
Zeroth law of thermodynamics P v t relationship in adiabatic process
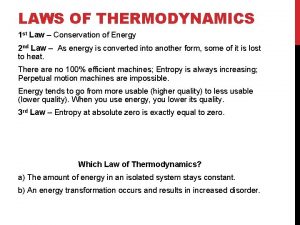
P v t relationship in adiabatic process Law of thermodynamics
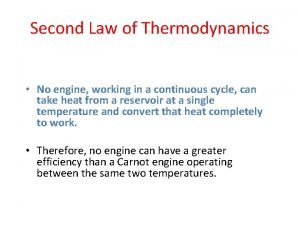
Law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Thermodynamics enthalpy of reaction and hess's law
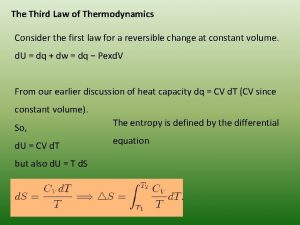
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
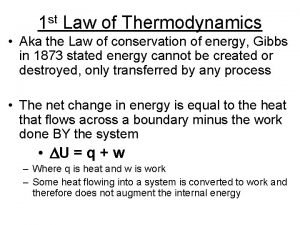
Third law of thermodynamics is depend on 1st law of thermodynamics
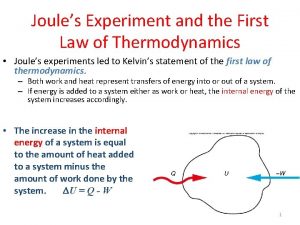
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics 2 nd law of thermodynamics
2 nd law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics Equation of second law of thermodynamics
Equation of second law of thermodynamics Thermodynamics
Thermodynamics Frist law of thermodynamics
Frist law of thermodynamics V=k/p
V=k/p Charles law constant
Charles law constant For today's meeting
For today's meeting Today meeting or today's meeting
Today meeting or today's meeting What is meeting and types of meeting
What is meeting and types of meeting What is meeting and types of meeting
What is meeting and types of meeting A first course in stochastic processes
A first course in stochastic processes Meeting traffic
Meeting traffic Kira & nadine first meeting video
Kira & nadine first meeting video Welcome to our first meeting
Welcome to our first meeting Welcome to our first meeting
Welcome to our first meeting Meeting jesus again for the first time
Meeting jesus again for the first time Narrow virtuosity examples
Narrow virtuosity examples First meeting of the year
First meeting of the year Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Chúa sống lại
Chúa sống lại Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V. c c
V. c c Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Số nguyên là gì
Số nguyên là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì