Thermodynamics www chemistry mut ac th12 thermodynamics ppt

![b) N 2(g) 3 + H 2(g) 2 NH 3(g) S rxn(2)] = ] b) N 2(g) 3 + H 2(g) 2 NH 3(g) S rxn(2)] = ]](https://slidetodoc.com/presentation_image_h/d06f78ca997b9f37369e76fd2629a318/image-15.jpg)

- Slides: 34

������ )Thermodynamics( ����� : www. chemistry. mut. ac. th/12 thermodynamics. ppt

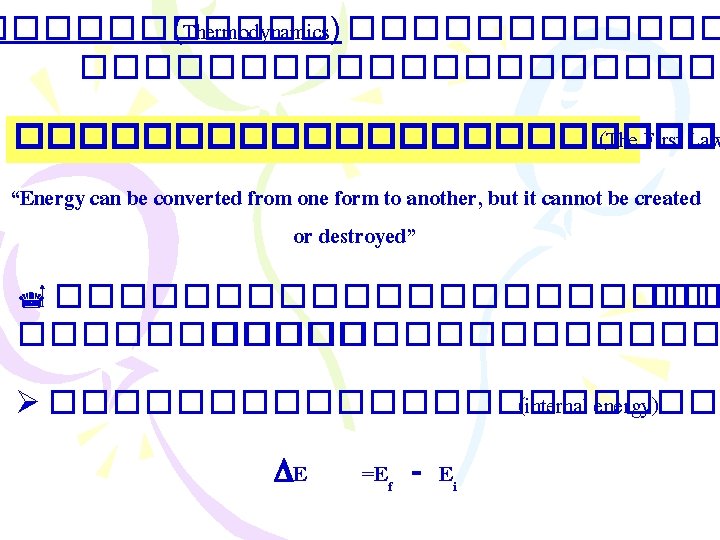
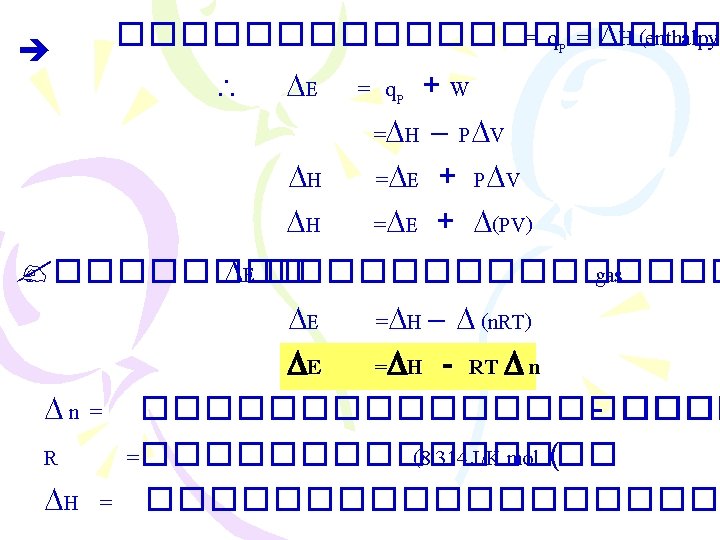
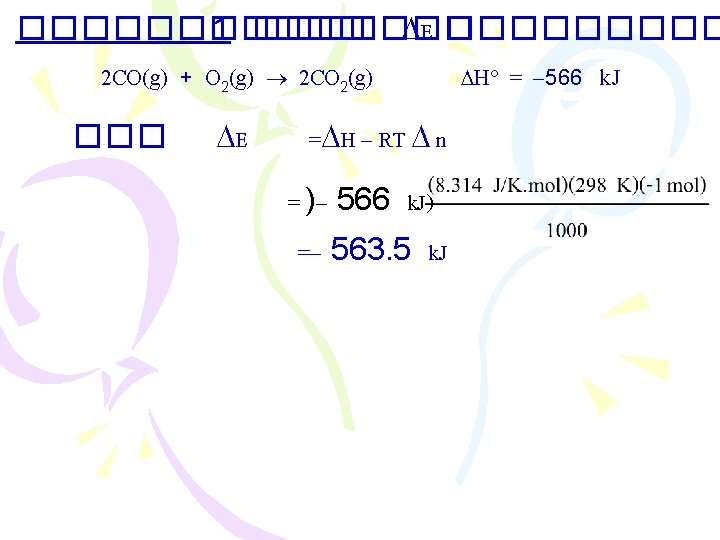
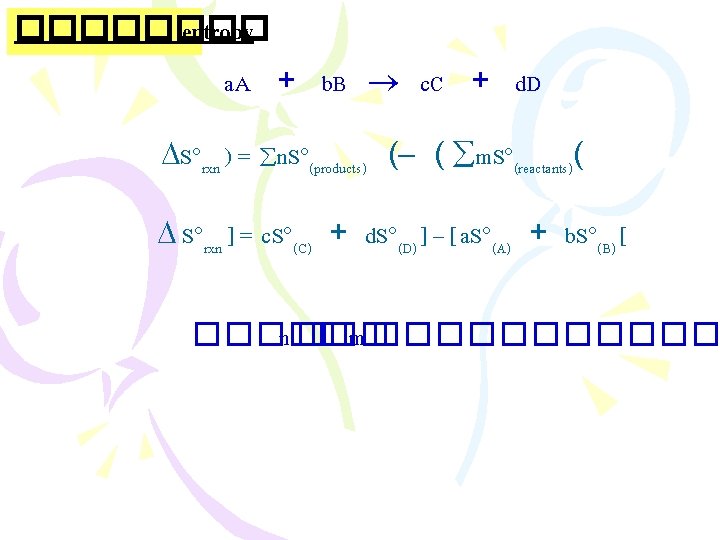
������ (Thermodynamics) ����������������� (The First Law “Energy can be converted from one form to another, but it cannot be created or destroyed” ����������� �������� Ø ����������� (internal energy) E =Ef - Ei



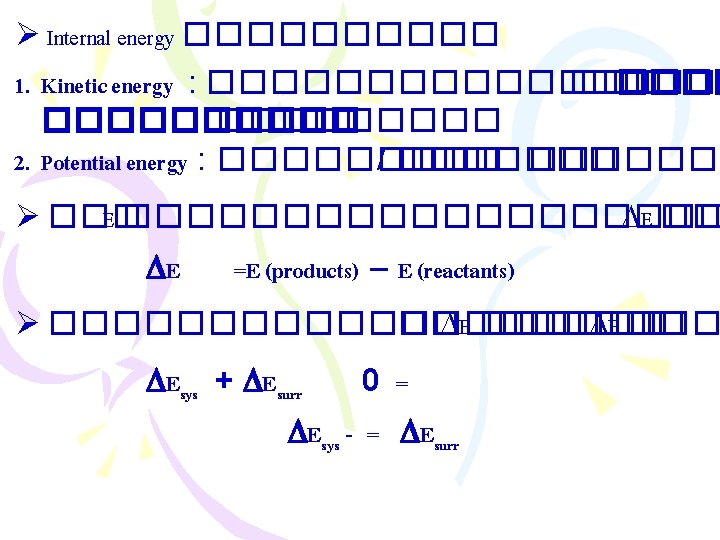
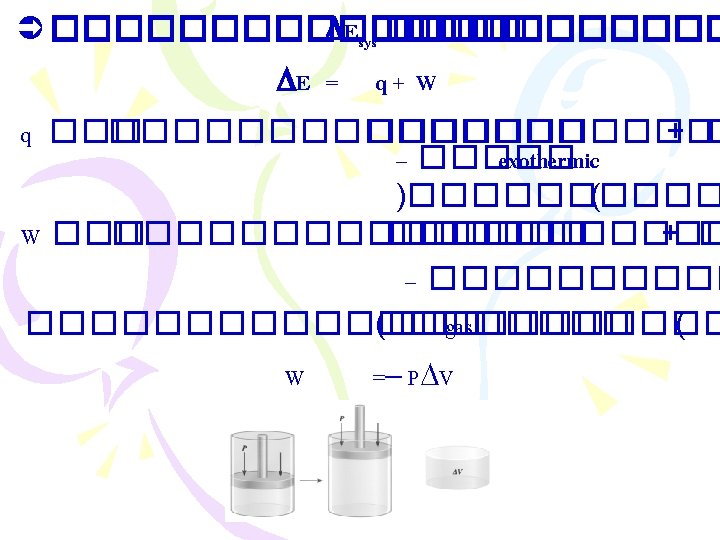
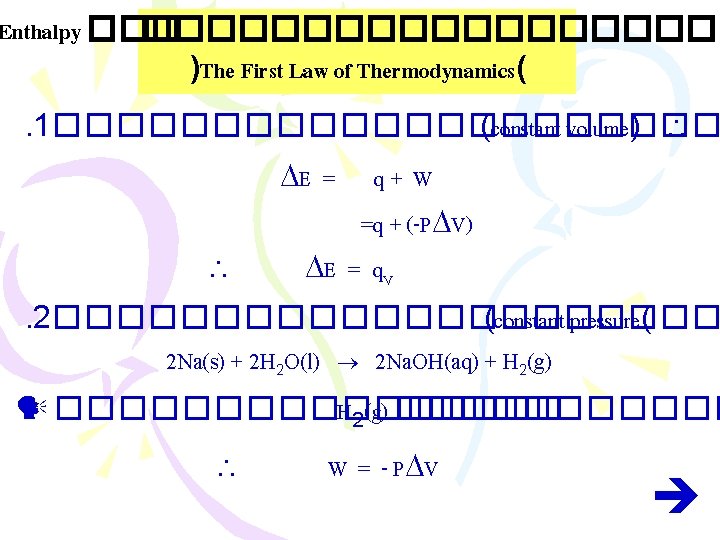
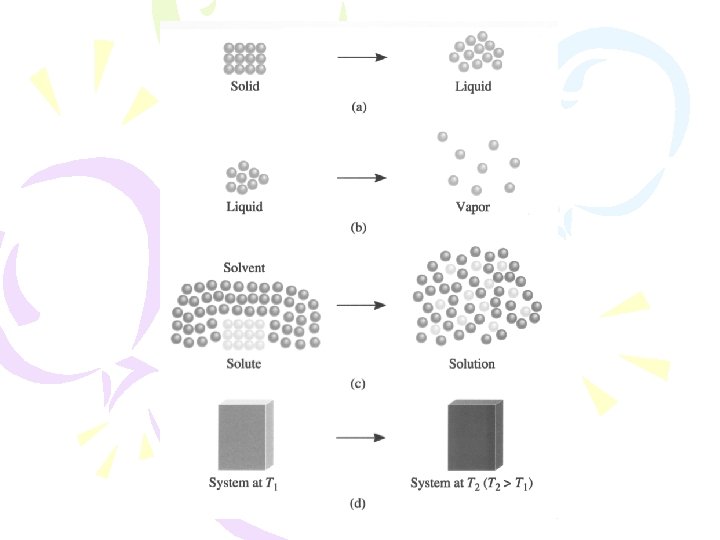
Enthalpy ����������� )The First Law of Thermodynamics( . 1����������� (constant volume) E = q+ W =q + (-P V) E = q. V . 2����������� (constant pressure( 2 Na(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g) �������� H 2(g) ������ W = - P V




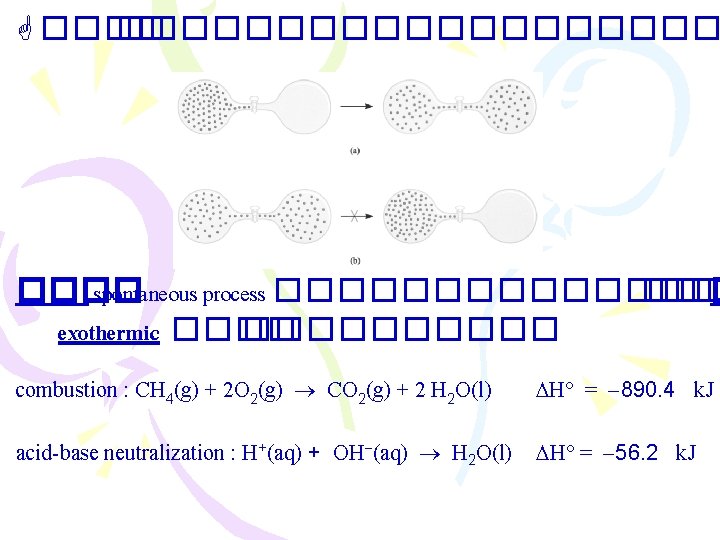
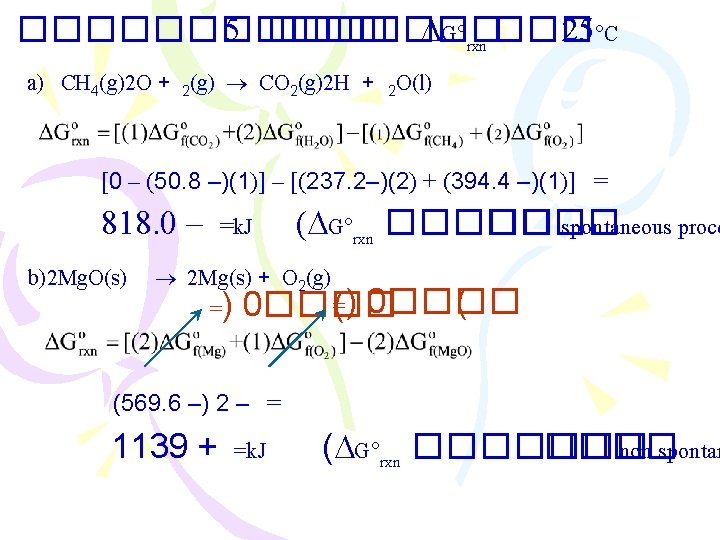
������������ spontaneous process ������� ��� � exothermic ���������� combustion : CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) H = 890. 4 k. J acid-base neutralization : H+(aq) + OH (aq) H 2 O(l) H = 56. 2 k. J





![b N 2g 3 H 2g 2 NH 3g S rxn2 b) N 2(g) 3 + H 2(g) 2 NH 3(g) S rxn(2)] = ]](https://slidetodoc.com/presentation_image_h/d06f78ca997b9f37369e76fd2629a318/image-15.jpg)
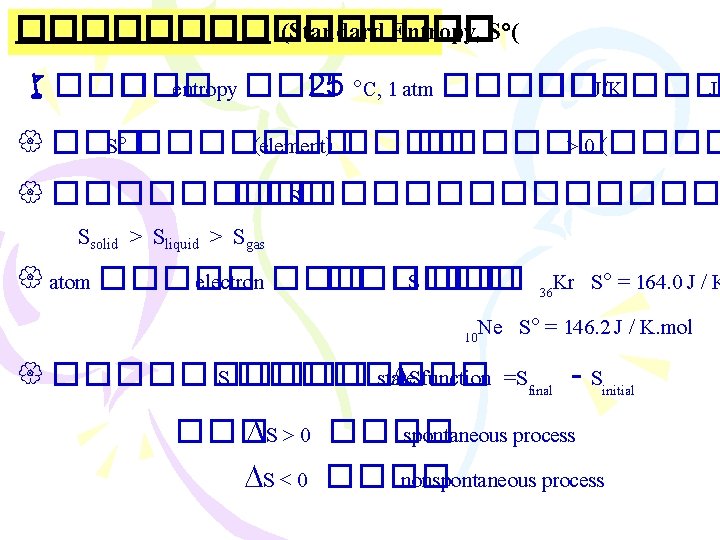
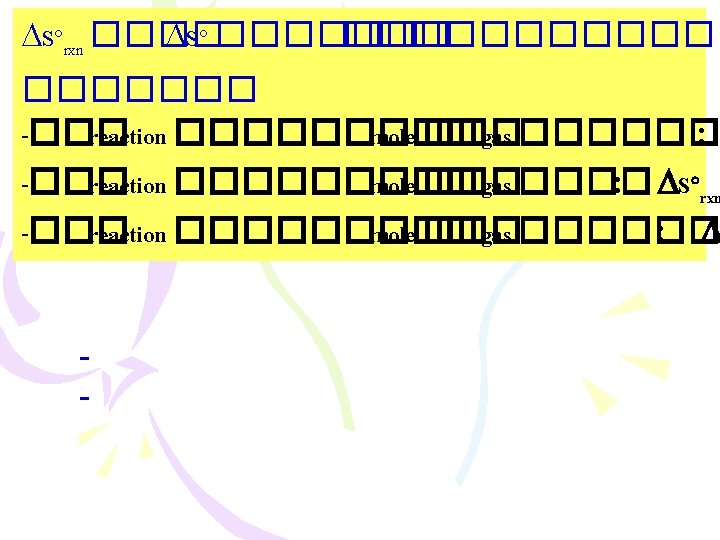
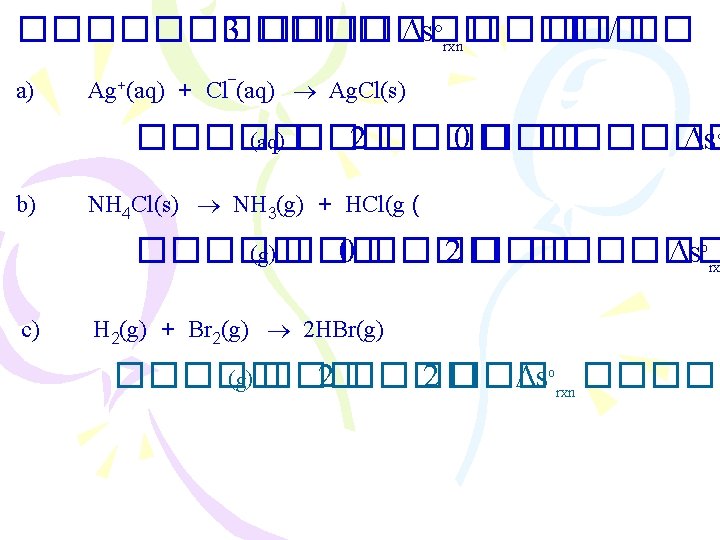
b) N 2(g) 3 + H 2(g) 2 NH 3(g) S rxn(2)] = ] – [(1) (3) + ] [(131)(3) + (192)(1)] – [(193)(2)] = 199 – =J/K c) H 2(g) + Cl 2(g) 2 HCl(g) S rxn(2)] = ] – [(1) (3) + [(223)(1) + (131)(1)] – (187)(2) = 20 + =J/K [



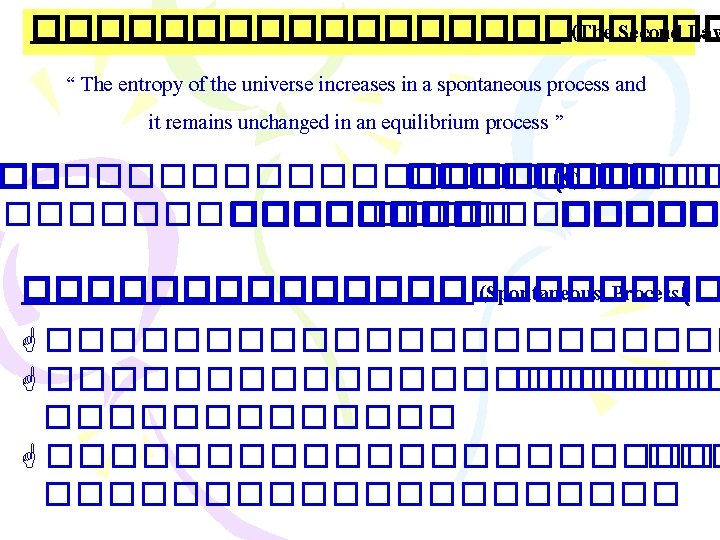
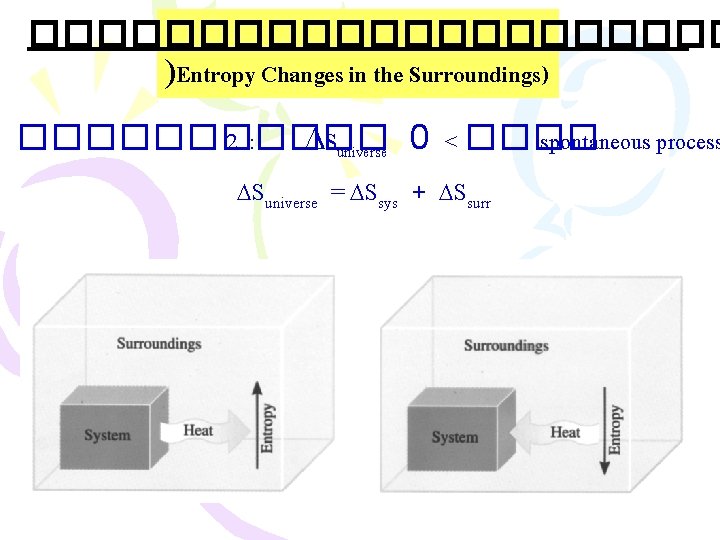
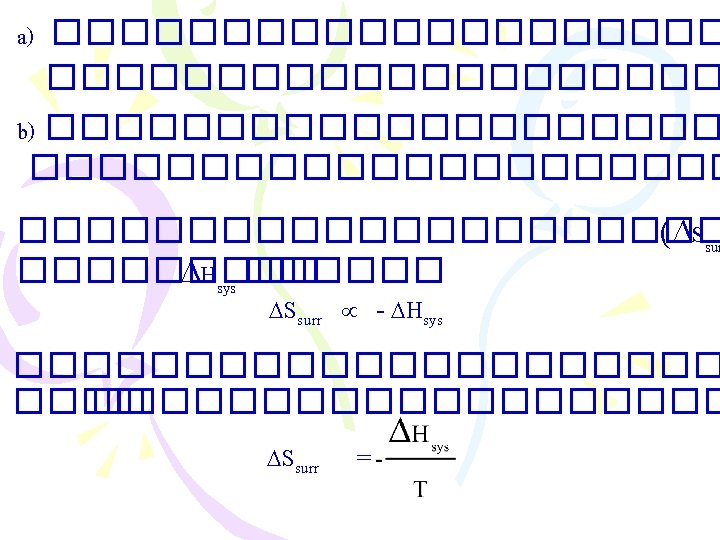
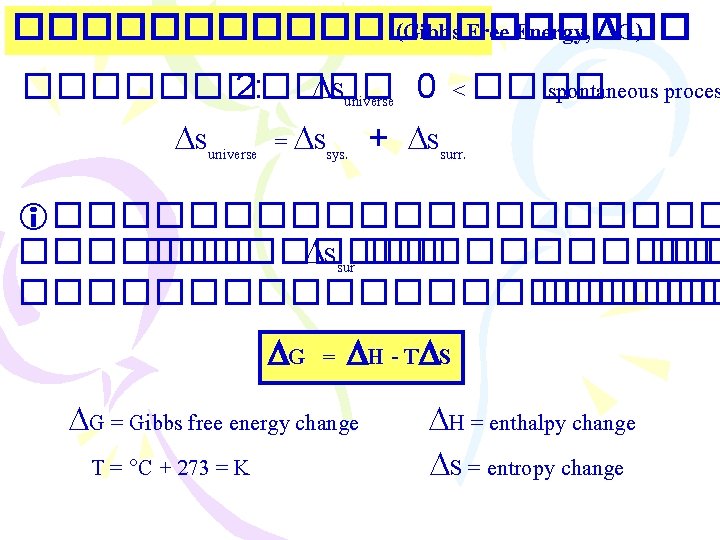
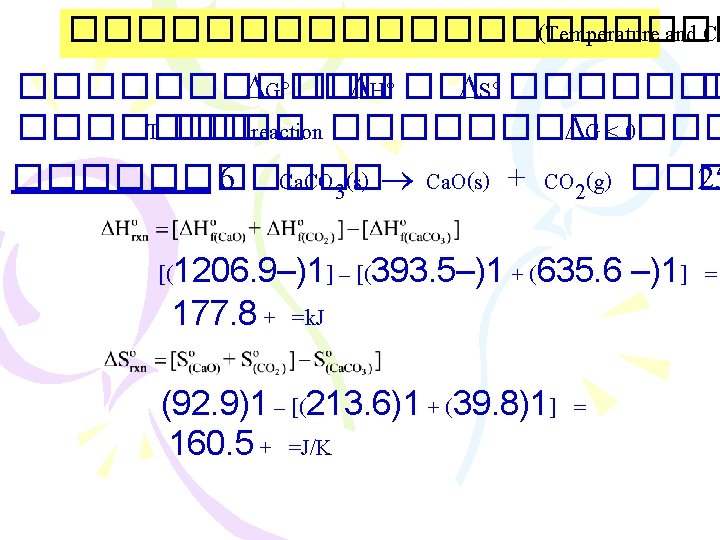
����������� )Entropy Changes in the Surroundings) ������ 2 : Suniverse 0 < ���� spontaneous process Suniverse = Ssys + Ssurr .


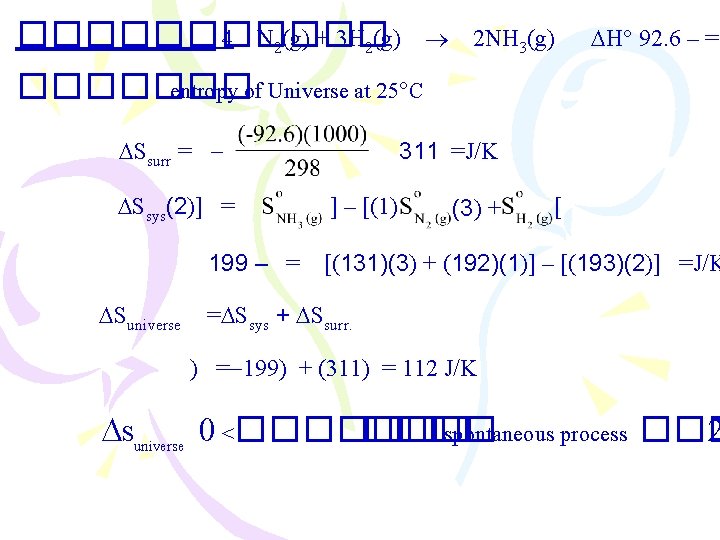
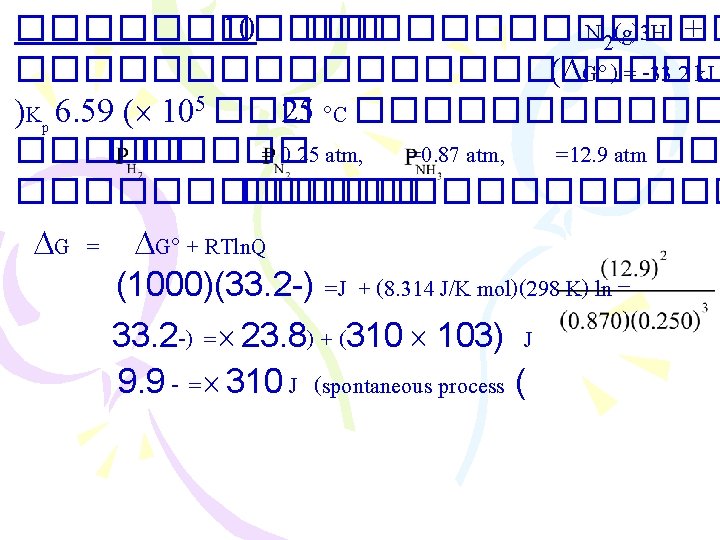
������ 4 N 2(g) + 3 H 2(g) 2 NH 3(g) H 92. 6 – = ������� entropy of Universe at 25 C Ssurr = Ssys(2)] = 199 – = Suniverse 311 =J/K ] – [(1) (3) + [ [(131)(3) + (192)(1)] – [(193)(2)] =J/K = Ssys + Ssurr. ) = 199) + (311) = 112 J/K Suniverse 0 <������� spontaneous process ��� 2

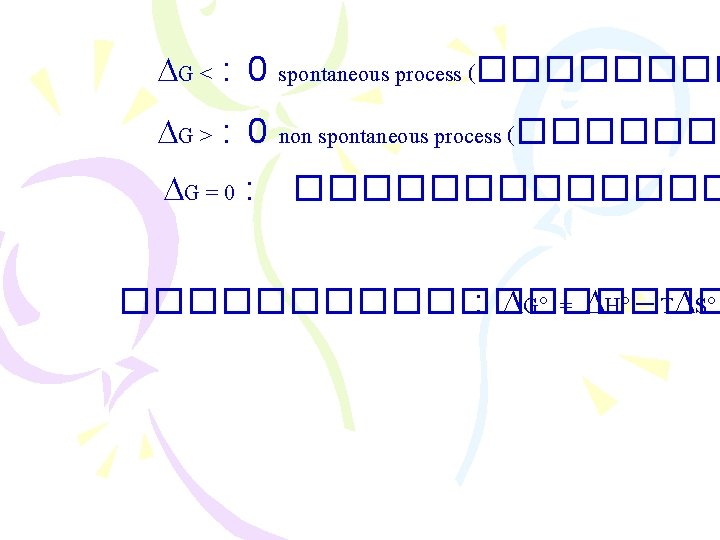
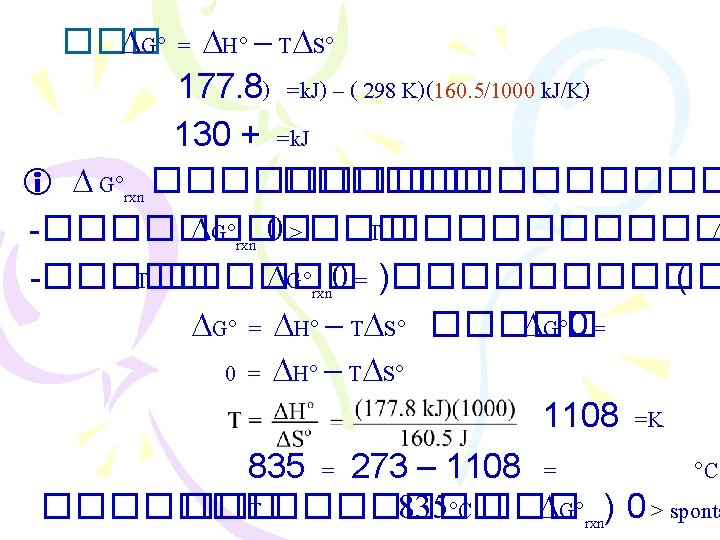
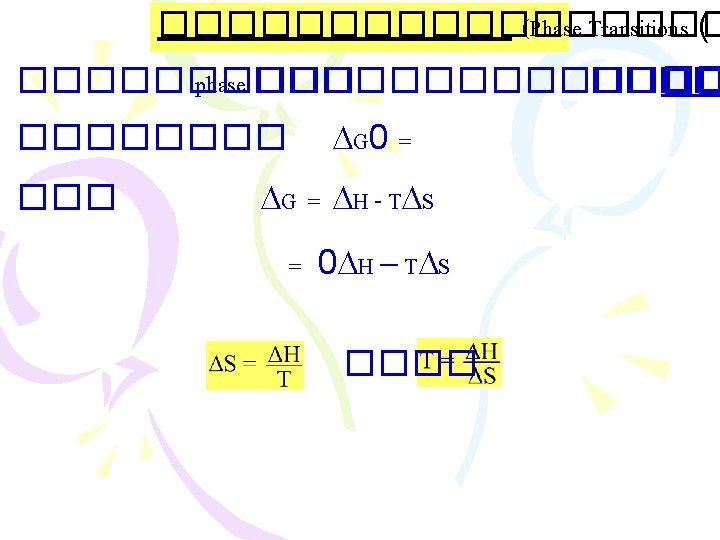
���������� (Gibbs Free Energy, G) ������ 2: Suniverse 0 < ���� spontaneous proces Suniverse = Ssys. + Ssurr. ���������� Ssur ������������ G = H - T S G = Gibbs free energy change T = C + 273 = K H = enthalpy change S = entropy change


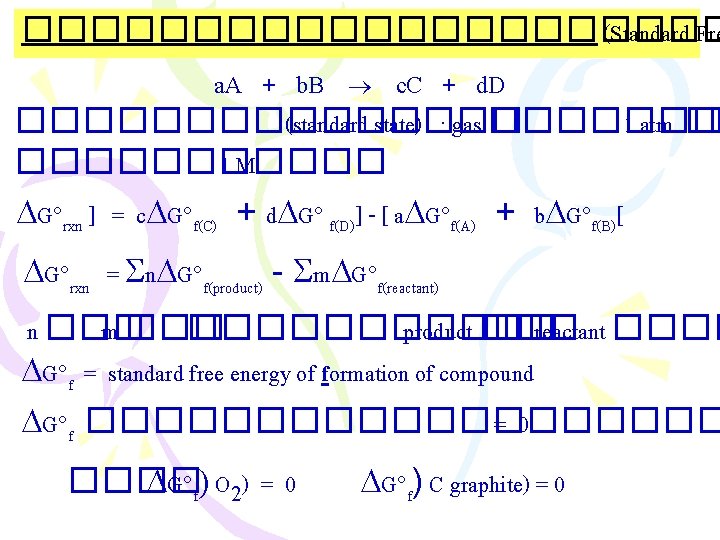
����������� (Standard Fre a. A + b. B c. C + d. D �������� (standard state) : gas ������� 1 atm �� ������ 1 M G rxn ] = c G f(C) + d G f(D)] - [ a G f(A) + b G f(B)[ G rxn = n G f(product) - m G f(reactant) n ��� m ������� product ��� reactant ���� G f = standard free energy of formation of compound G f ���������� =0 ���� G f) O 2) = 0 G f) C graphite) = 0





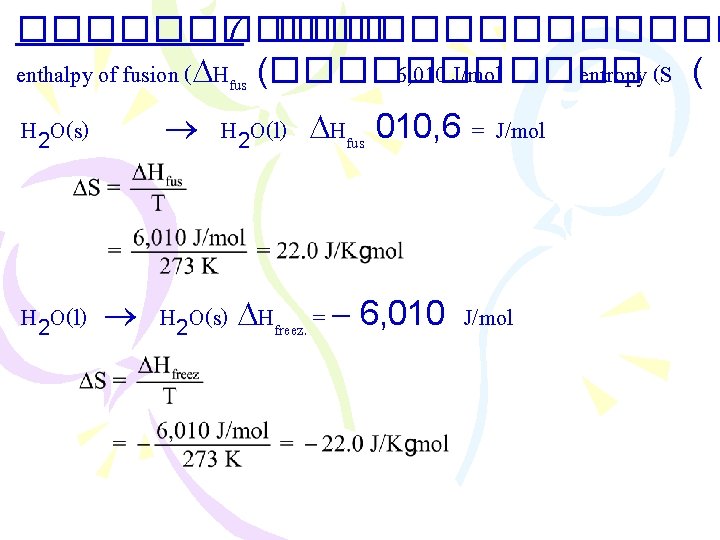
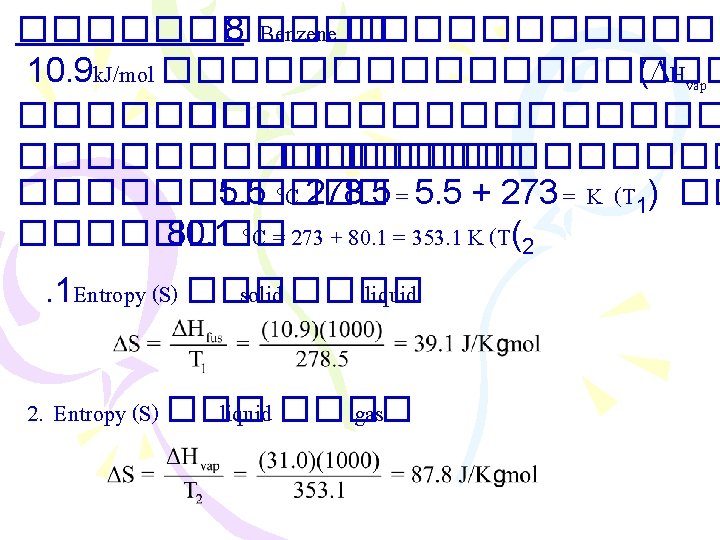
������ 7 ������� enthalpy of fusion ( Hfus (������� 6, 010 J/mol ���� entropy (S ( H 2 O(s) H 2 O(l) Hfus 010, 6 = J/mol H 2 O(l) H 2 O(s) Hfreez. = 6, 010 J/mol


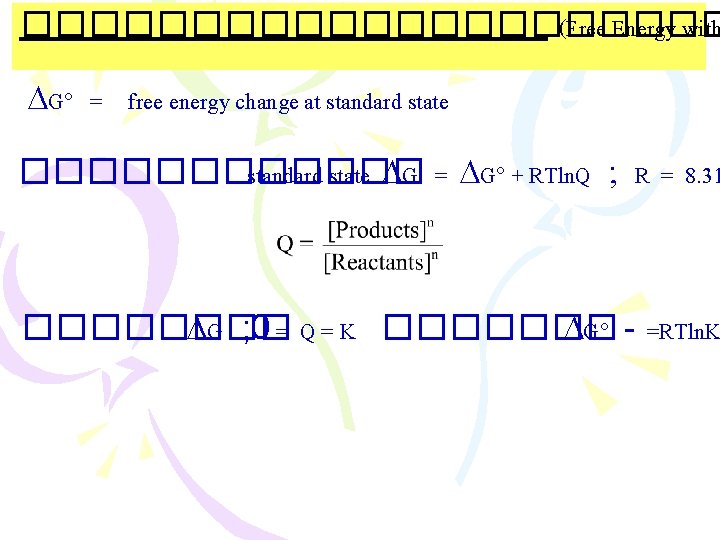
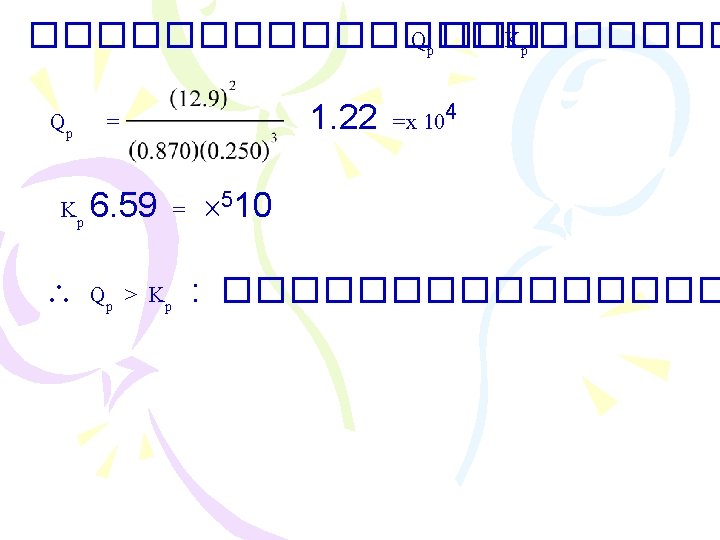
����������� (Free Energy with G = free energy change at standard state ������ standard state G = G + RTln. Q ; R = 8. 31 ���� G ; 0 = Q = K ������� G - =RTln. K

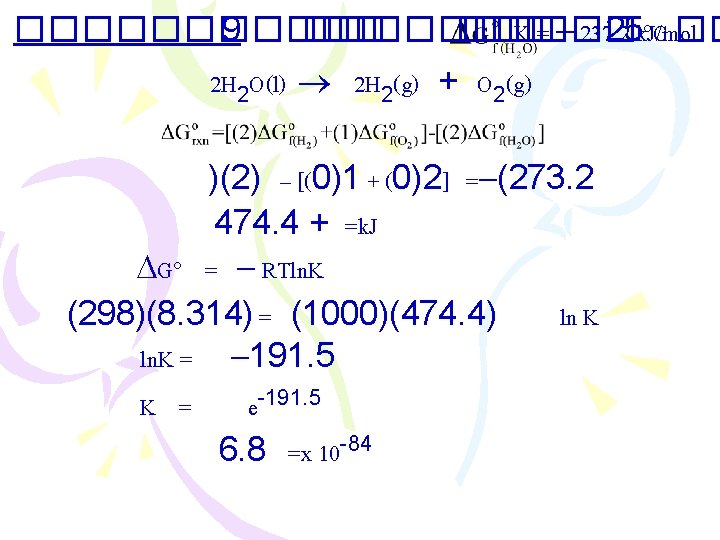
������ 9 ������� K ��� 25 k. J/mol C �� = 237. 2 2 H 2 O(l) 2 H 2(g) + O 2(g) )(2) – [(0)1 + (0)2] = (273. 2 474. 4 + =k. J G = RTln. K (298)(8. 314) = (1000)(474. 4) ln K ln. K = 191. 5 K = e-191. 5 6. 8 =x 10 -84



����������� (The Third La “The entropy of a perfect crystalline substance is zero at the absolute zero of temperature” ����������� (0 K (