Mass Mass A measurement of the amount of























- Slides: 42

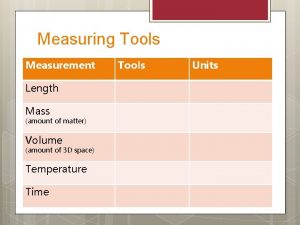
Mass

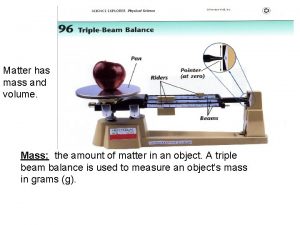
Mass: A measurement of the amount of matter in an object It can be measured with a scale or balance It is measured in grams.

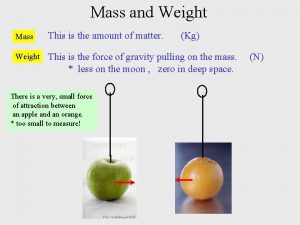
Weight: How heavy or light an object is based on its mass, OR The force of Gravity on an object. (Gravity: the force that pulls all things to the center of the Earth)

Weight can change depending on where you are in the universe, different planets have different gravities! The weight may change planet to planet, but the mass will not!

Volume

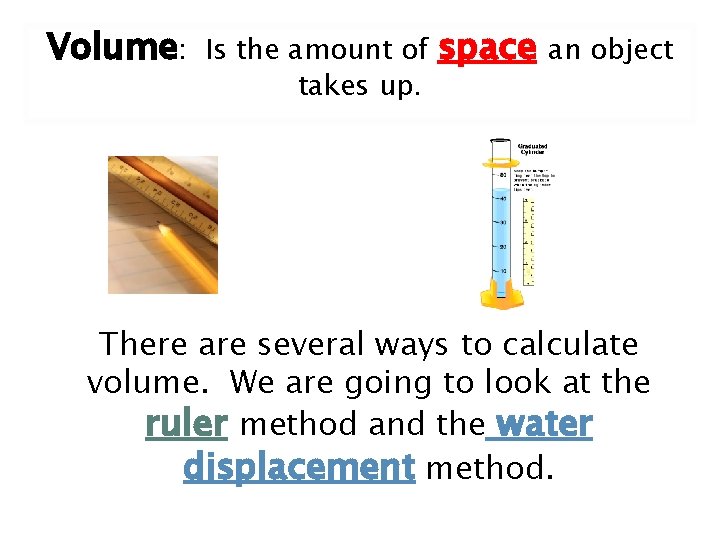
Volume: Is the amount of takes up. space an object There are several ways to calculate volume. We are going to look at the ruler method and the water displacement method.

So how do we find the volume of an object using a ruler?

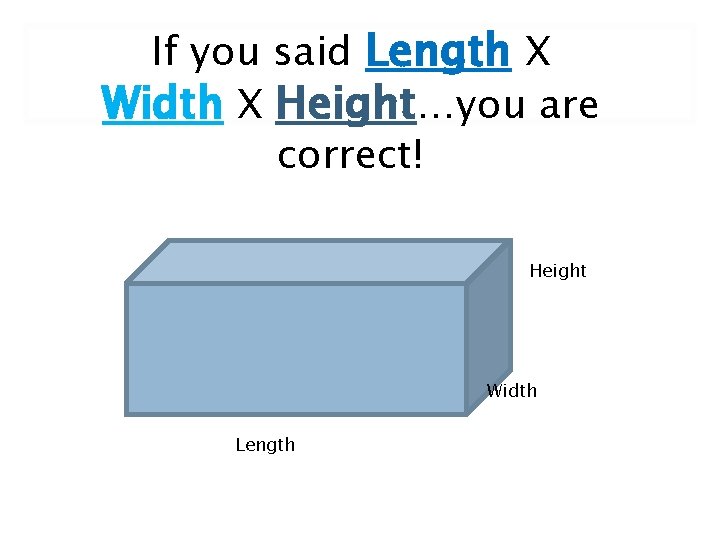
If you said Length X Width X Height…you are correct! Height Width Length

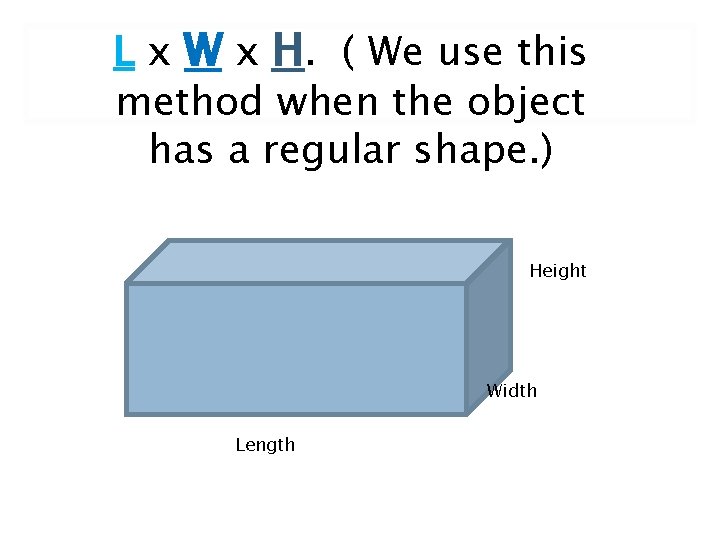
L x W x H. ( We use this method when the object has a regular shape. ) Height Width Length

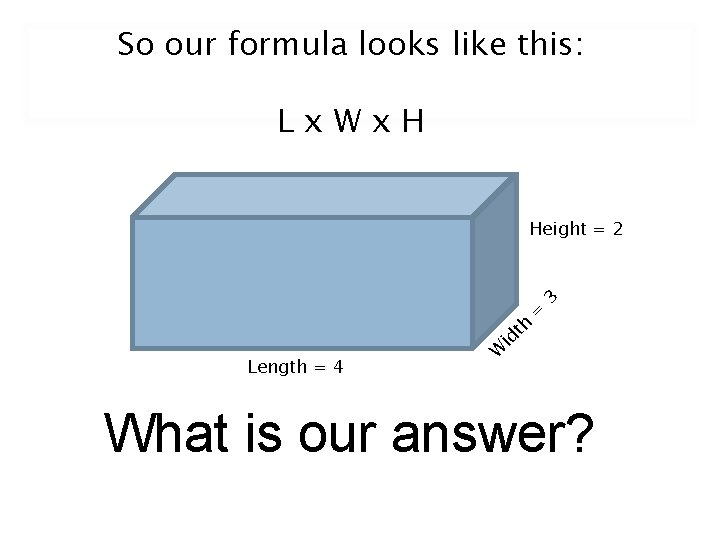
So our formula looks like this: Lx. Wx. H Length = 4 W id th = 3 Height = 2 What is our answer?

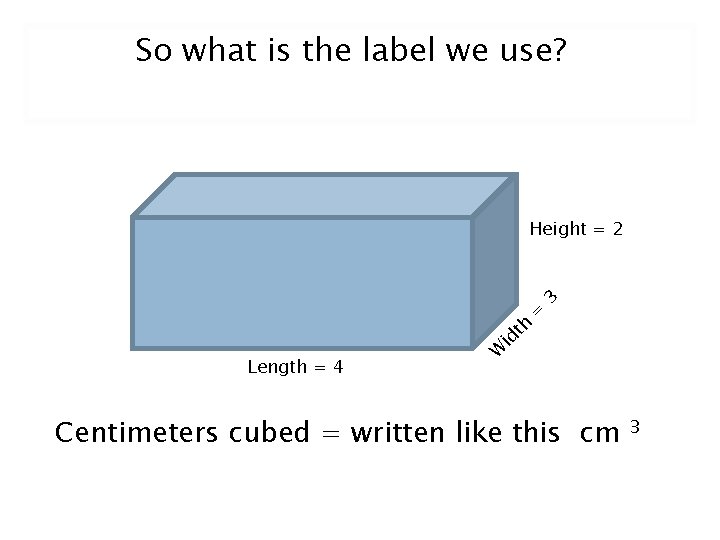
So what is the label we use? Length = 4 W id th = 3 Height = 2 Centimeters cubed = written like this cm 3

How many of you have every gotten into the tub only to find out you have filled it too high and water flows over the top?

How does it work?

Just like when you got into the tub, the water level had to go up in proportion to the amount of space you were taking up.

So would be the first step?

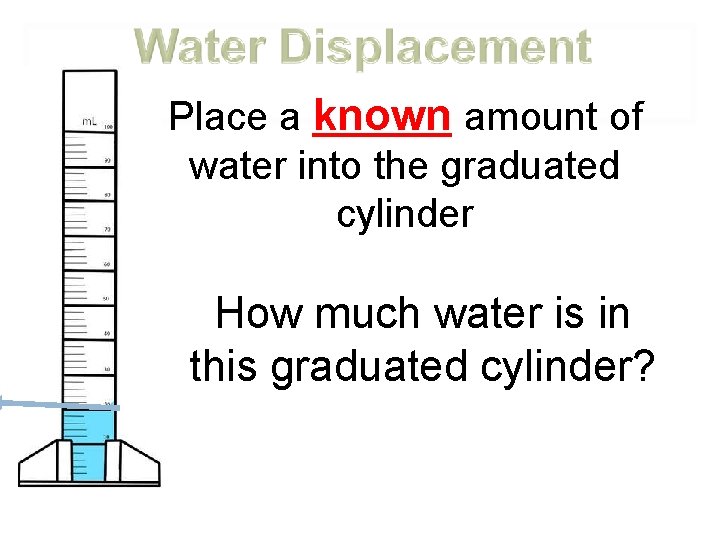
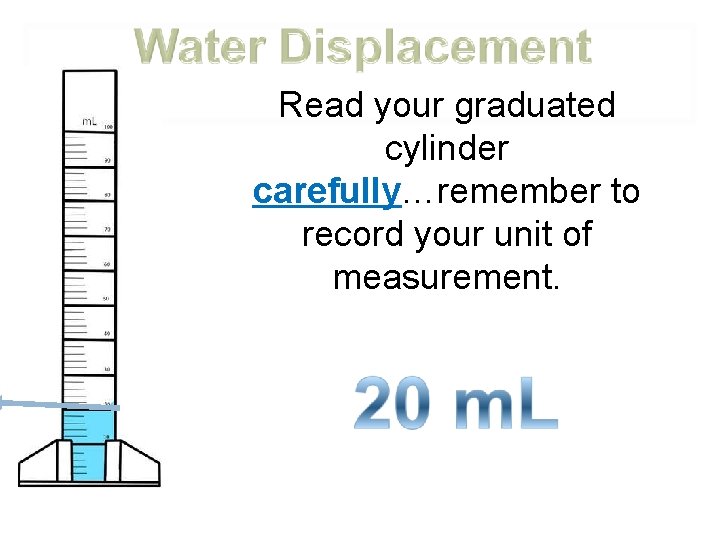
Place a known amount of water into the graduated cylinder How much water is in this graduated cylinder?

Read your graduated cylinder carefully…remember to record your unit of measurement.

Very carefully place your object into the graduated cylinder

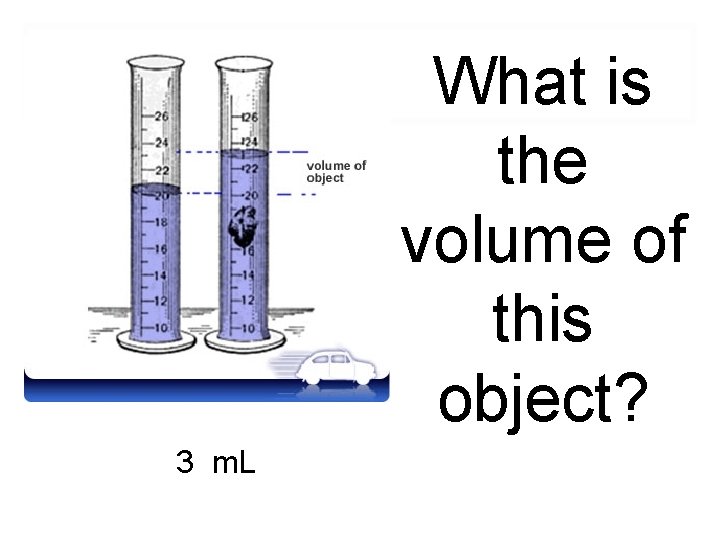
Read the volume of the water after the object has been put in, then…

subtract your beginning water level from you ending water level. 25 m. L 20 m. L 5 m. L Ending Water Level -Beginning Water Level Volume of the object

Now what is the unit of measurement we will be using with our answer.

What is the volume of this object? 3 m. L

DENSITY

WHAT IS THE DIFFERENCE?

MASS, VOLUME & DENSITY • Mass and volume are physical properties. Together they are used to find the DENSITY of an object! • What is Volume? • What is Mass?

WHAT IS DENSITY? • Density is a comparison of how much matter there is in a certain amount of space. • The amount of matter per unit of space • More matter in less space= more dense • Less matter in more space= less dense

WHAT DOES THAT REALLY MEAN? • How tightly packed the matter in an object is

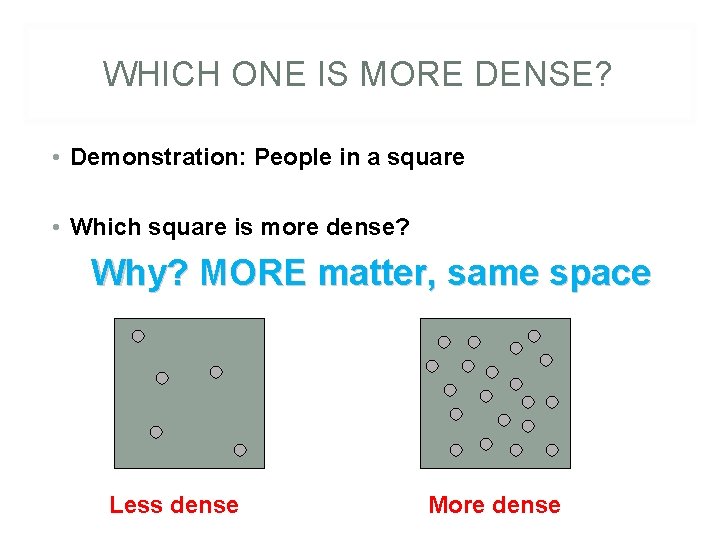
WHICH ONE IS MORE DENSE? • Demonstration: People in a square • Which square is more dense? Why? MORE matter, same space Less dense More dense

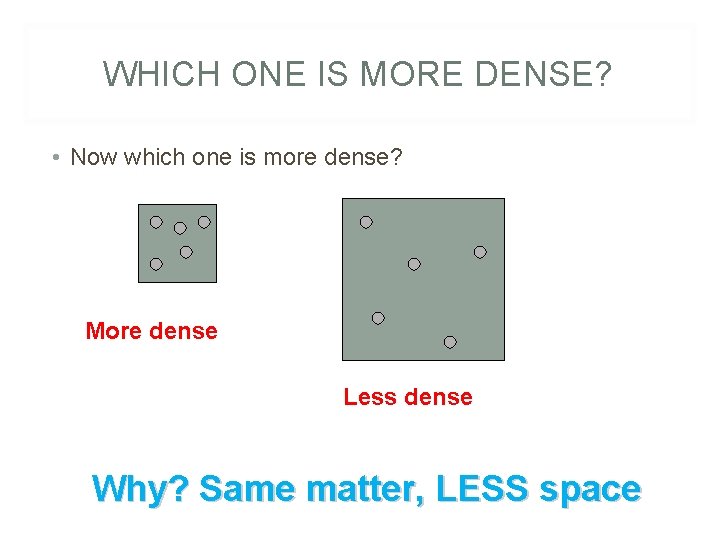
WHICH ONE IS MORE DENSE? • Now which one is more dense? More dense Less dense Why? Same matter, LESS space

YOU TRY • How do I make the front of the classroom MORE dense? LESS dense?

WHICH IS MORE DENSE? • Claim: Cylinder 2 is more dense. • Evidence: The matter is more tightly packed than cylinder 1 and there is more matter in the same amount of space. • Reasoning: Density is the amount of matter per unit of space. The more matter there is in a given space, the more dense an object is. Since cylinder 2 has more matter in the same amount of space, it is more dense.


DENSITY- LET’S CALCULATE!!!

UNITS FOR DENSITY • Density = mass volume OR mass ÷ volume. • Units for density: • g/cm 3 • g/m. L . • Why are these the units for density? ALWAYS REMEMBER UNITS!

LET’S TRY A DENSITY PROBLEM TOGETHER • Frank has a paper clip. It has a mass of 9 g and a volume of 3 cm 3. What is its density? • • D= m/v M= 9 g, V=3 cm 3 D= 9 g/3 cm 3 D= 3 g/cm 3 • Frank also has an eraser. It has a mass of 42 g, and a volume of 7 m. L. What is its density? • • D= m/v M= 42 g, V=7 m. L D= 42 g/7 m. L D= 6 g/m. L

PRACTICE TIME

EXIT TICKET 1) Osmium is a very dense metal. What is its density if 50 g of the metal occupies a volume of 2 cm 3? 2) Which object is the most dense? Why? • Object 1: 1. 4 g/m. L • Object 2: 7 g/m. L • Object 3: 0. 3 g/m. L • Object 4: 24 g/m. L • Object 5: 1. 04 g/m. L

WORK ON THESE PROBLEMS WITH YOUR NEIGHBOR. • Jack has a rock. The rock has a mass of 6 g and a volume of 3 cm 3. What is the density of the rock? • Jill has a gel pen. The gel pen has a mass of 8 g and a volume of 2 cm 3. What is the density of the rock?

CALCULATING DENSITY WITH WATER DISPLACEMENT

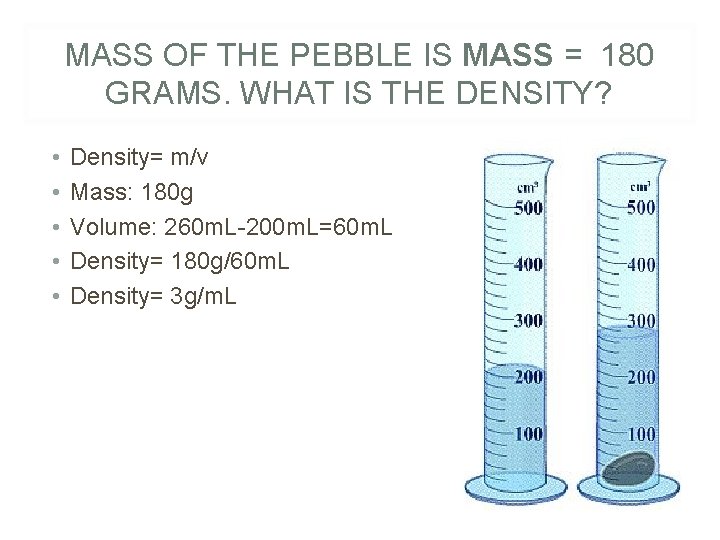
MASS OF THE PEBBLE IS MASS = 180 GRAMS. WHAT IS THE DENSITY? • • • Density= m/v Mass: 180 g Volume: 260 m. L-200 m. L=60 m. L Density= 180 g/60 m. L Density= 3 g/m. L

EXIT QUESTION • The mass of an object is 90 grams. You fill a graduated cylinder with 20 m. L of water. You drop the object into the graduated cylinder and the water rises to 25 m. L. What is the density of the object?

SUPER SCIENTIST QUESTION OF THE DAY • Jake has a book, a ruler, and a balance. • How can Jake find the density of the book with the tools he has?
 Dmv triangle
Dmv triangle Si unit ladder
Si unit ladder Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Bổ thể
Bổ thể Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Phản ứng thế ankan
Phản ứng thế ankan Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Chúa yêu trần thế
Chúa yêu trần thế điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Hệ hô hấp
Hệ hô hấp Công của trọng lực
Công của trọng lực Số.nguyên tố
Số.nguyên tố đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Tư thế ngồi viết
Tư thế ngồi viết Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Gấu đi như thế nào
Gấu đi như thế nào Thể thơ truyền thống
Thể thơ truyền thống Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là đại từ thay thế
đại từ thay thế Ng-html
Ng-html Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau 101012 bằng
101012 bằng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Glasgow thang điểm
Glasgow thang điểm