Mass vs Weight Mass depends on the amount



- Slides: 10

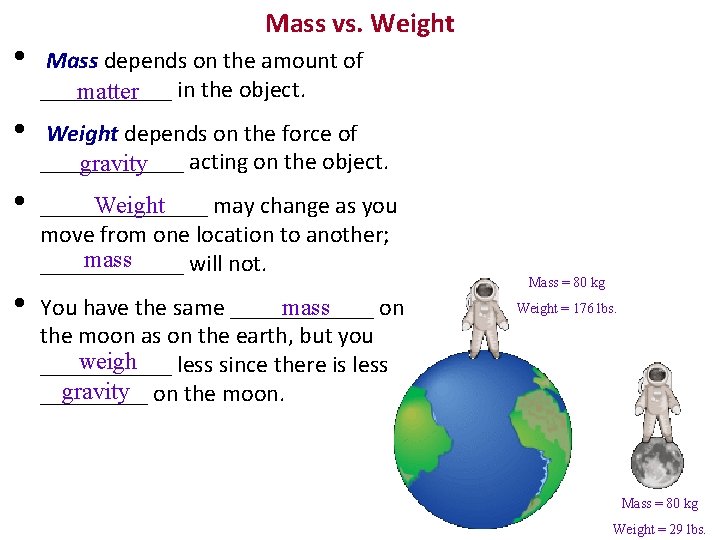
Mass vs. Weight • Mass depends on the amount of ______ in the object. matter • Weight depends on the force of ______ acting on the object. gravity • Weight _______ may change as you move from one location to another; mass ______ will not. • You have the same ______ on mass the moon as on the earth, but you weigh ______ less since there is less gravity on the moon. _____ Mass = 80 kg Weight = 176 lbs. Mass = 80 kg Weight = 29 lbs.

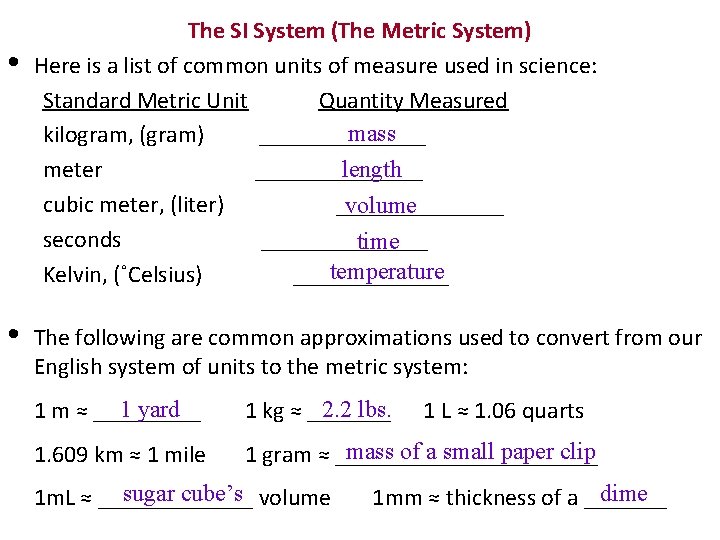
• The SI System (The Metric System) Here is a list of common units of measure used in science: Standard Metric Unit Quantity Measured mass kilogram, (gram) _______ meter cubic meter, (liter) seconds Kelvin, (˚Celsius) • length ______________ volume _______ time temperature _______ The following are common approximations used to convert from our English system of units to the metric system: 1 yard 1 m ≈ _____ 2. 2 lbs. 1 kg ≈ _______ 1. 609 km ≈ 1 mile mass of a small paper clip 1 gram ≈ ___________ sugar cube’s volume 1 m. L ≈ _______ 1 L ≈ 1. 06 quarts dime 1 mm ≈ thickness of a _______


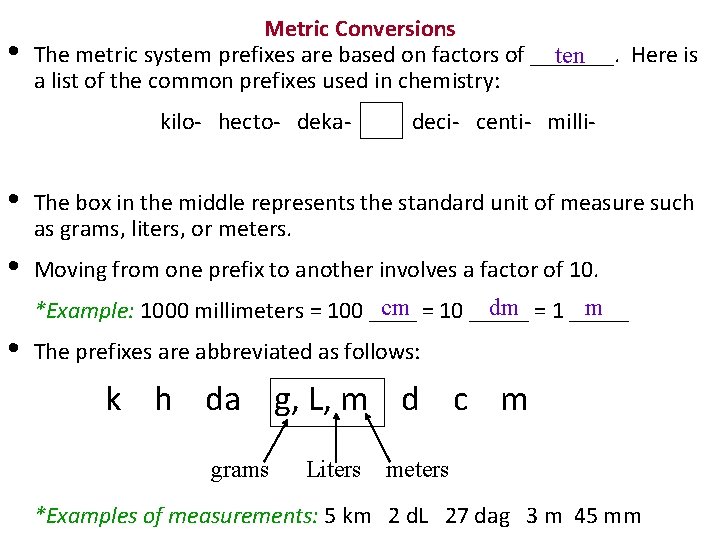
• Metric Conversions The metric system prefixes are based on factors of _______. Here is ten a list of the common prefixes used in chemistry: kilo- hecto- deka- deci- centi- milli- • The box in the middle represents the standard unit of measure such as grams, liters, or meters. • Moving from one prefix to another involves a factor of 10. cm = 10 _____ dm = 1 _____ m *Example: 1000 millimeters = 100 ____ • The prefixes are abbreviated as follows: k h da g, L, m d c m grams Liters meters *Examples of measurements: 5 km 2 d. L 27 dag 3 m 45 mm

Metric Conversions • To convert from one prefix to another, simply count how many places you move on the scale above, and that is the same # of places the decimal point will move in the same direction. deci- centi- milli. Practice Problems: kilo- hecto- deka 380, 000 0. 00145 380 km = _______m 1. 45 mm = _____m 4. 61 0. 0004 461 m. L = ______d. L 0. 4 cg = ______ dag 0. 26 g =_______ mg 230, 000 m = _______km 260 230 Other Metric Equivalents 1 m. L = 1 cm 3 1 L = 1 dm 3 For water only: 1 L = 1 dm 3 = 1 kg of water or 1 m. L = 1 cm 3 = 1 g of water Practice Problems: 0. 3 L (1) How many liters of water are there in 300 cm 3 ? ______ 50 kg (2) How many kg of water are there in 500 d. L? _______

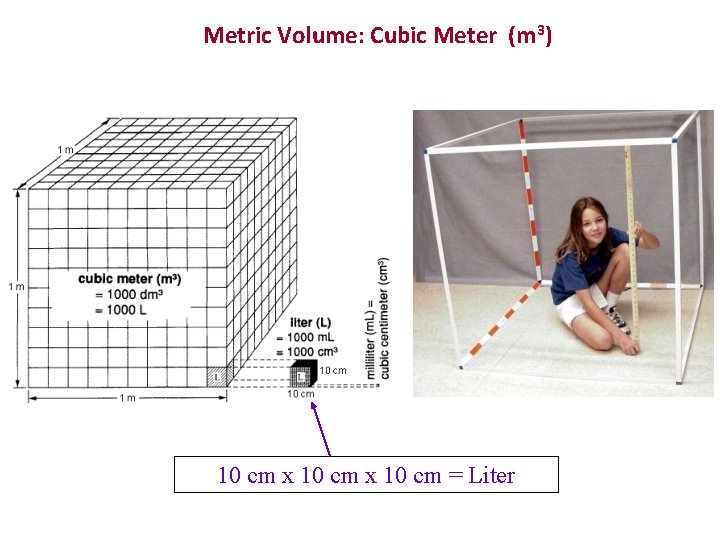
Metric Volume: Cubic Meter (m 3) 10 cm x 10 cm = Liter

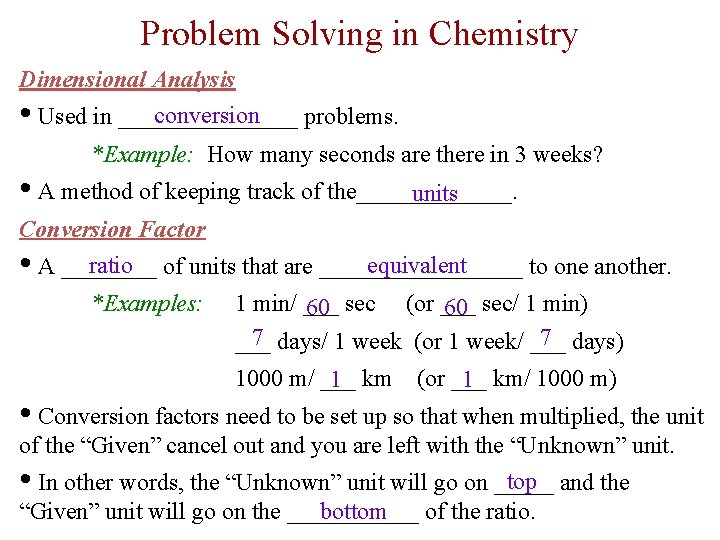
Problem Solving in Chemistry Dimensional Analysis conversion • Used in ________ problems. *Example: How many seconds are there in 3 weeks? • A method of keeping track of the_______. units Conversion Factor ratio of units that are _________ equivalent • A ____ to one another. *Examples: 1 min/ ___ 60 sec (or ___ 60 sec/ 1 min) 7 days/ 1 week (or 1 week/ ___ 7 days) ___ 1000 m/ ___ 1 km (or ___ 1 km/ 1000 m) • Conversion factors need to be set up so that when multiplied, the unit of the “Given” cancel out and you are left with the “Unknown” unit. top and the • In other words, the “Unknown” unit will go on _____ bottom “Given” unit will go on the ______ of the ratio.

How to Use Dimensional Analysis to Solve Conversion Problems • Step 1: Given Identify the “____”. This is typically the only number given in the problem. This is your starting point. Write it down! Then write “x _____”. This will be the first conversion factor ratio. • Step 2: Identify the “______”. This is what are you trying to Unknown figure out. • Step 3: Identify the ______ Sometimes you will conversion _____. factors simply be given them in the problem ahead of time. • Step 4: By using these conversion factors, begin planning a solution to convert from the given to the unknown. • Step 5: When your conversion factors are set up, _____ multiply all the divide numbers on top of your ratios, and ______ by all the numbers on bottom. If your units did not ____ cancel ______ out correctly, you’ve messed up!

Practice Problems: (1)How many hours are there in 3. 25 days? 3. 25 days x 24 hrs = 78 hrs 1 day (2) How many yards are there in 504 inches? 504 in. x 1 ft 12 in. x 1 yard = 14 yards 3 ft (3) How many days are there in 26, 748 seconds? 26, 748 sec x 1 min x 1 hr x 1 day 60 sec 60 min 24 hrs = 0. 30958 days

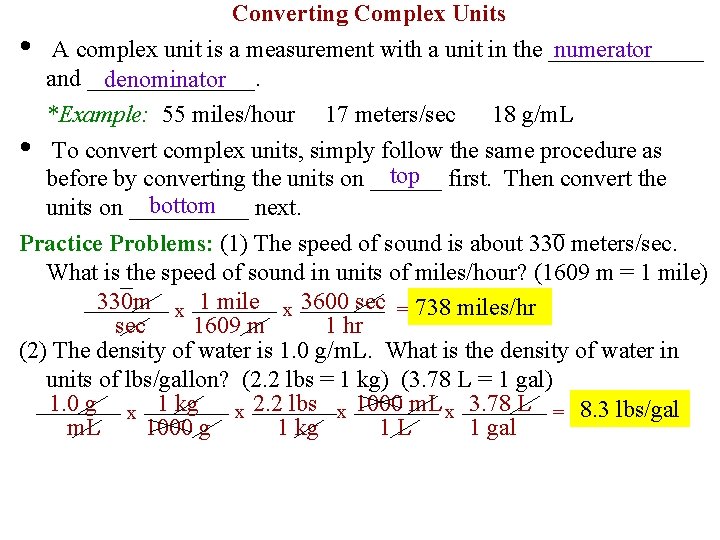
• Converting Complex Units numerator A complex unit is a measurement with a unit in the _______ and _______. denominator *Example: 55 miles/hour 17 meters/sec 18 g/m. L • To convert complex units, simply follow the same procedure as top first. Then convert the before by converting the units on ______ bottom units on _____ next. Practice Problems: (1) The speed of sound is about 330 meters/sec. What is the speed of sound in units of miles/hour? (1609 m = 1 mile) 330 m x 1 mile x 3600 sec = 738 miles/hr sec 1609 m 1 hr (2) The density of water is 1. 0 g/m. L. What is the density of water in units of lbs/gallon? (2. 2 lbs = 1 kg) (3. 78 L = 1 gal) 1. 0 g x 1 kg x 2. 2 lbs x 1000 m. L x 3. 78 L = 8. 3 lbs/gal m. L 1000 g 1 kg 1 L 1 gal