Density the amount of mass a material has















- Slides: 36

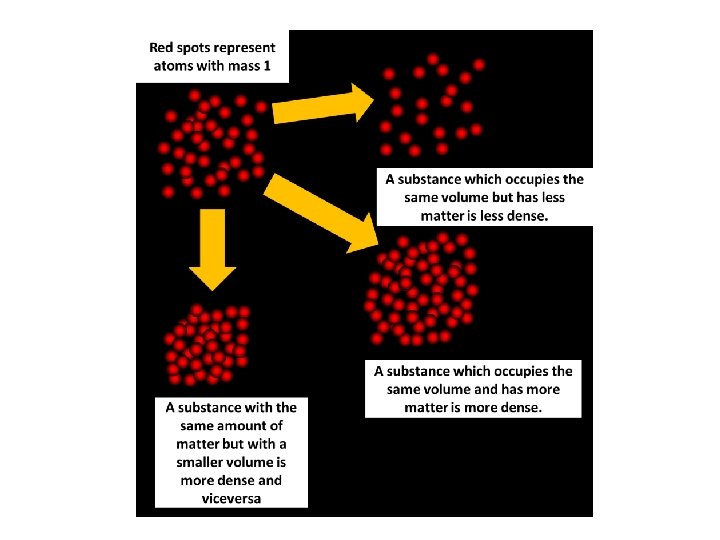
Density - the amount of mass a material has for a given volume

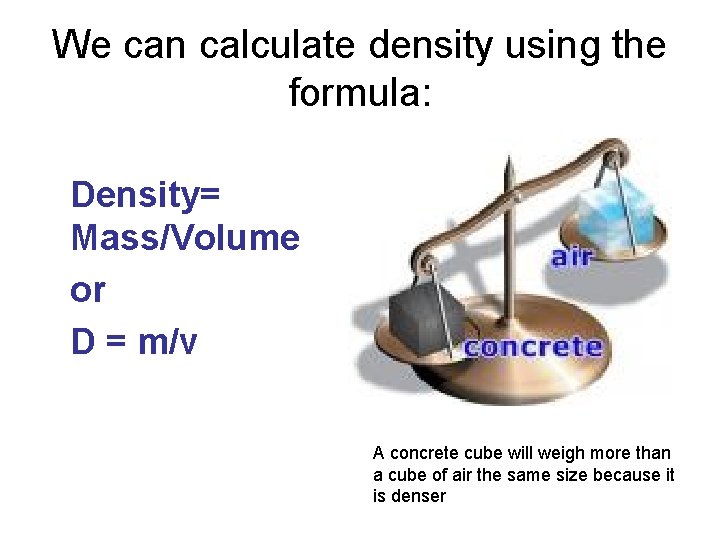
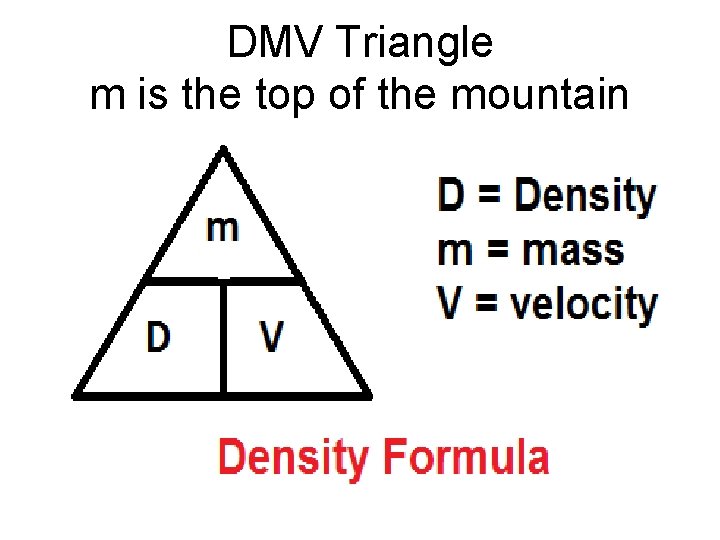
We can calculate density using the formula: Density= Mass/Volume or D = m/v . A concrete cube will weigh more than a cube of air the same size because it is denser

Song on density formula http: //www. youtube. com/watch? v=Vf. M DC 4 gu. XZg

Density is measured in: • • g/cm 3 kg/m 3 g/m. L kg/L

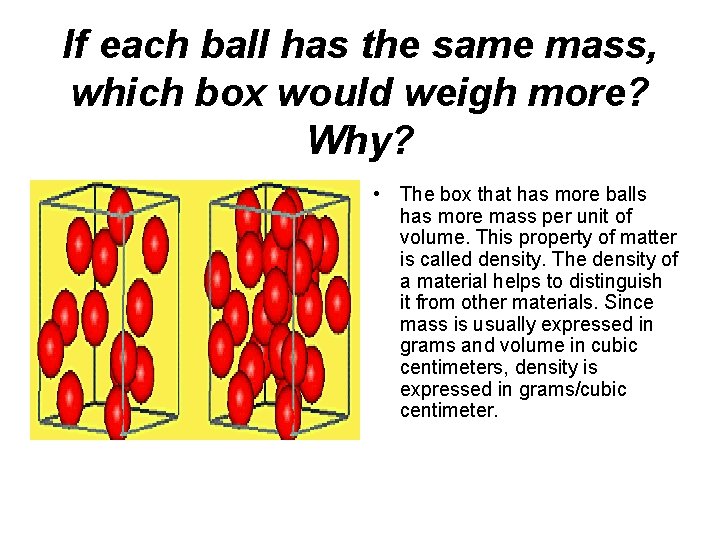
If each ball has the same mass, which box would weigh more? Why? • The box that has more balls has more mass per unit of volume. This property of matter is called density. The density of a material helps to distinguish it from other materials. Since mass is usually expressed in grams and volume in cubic centimeters, density is expressed in grams/cubic centimeter.

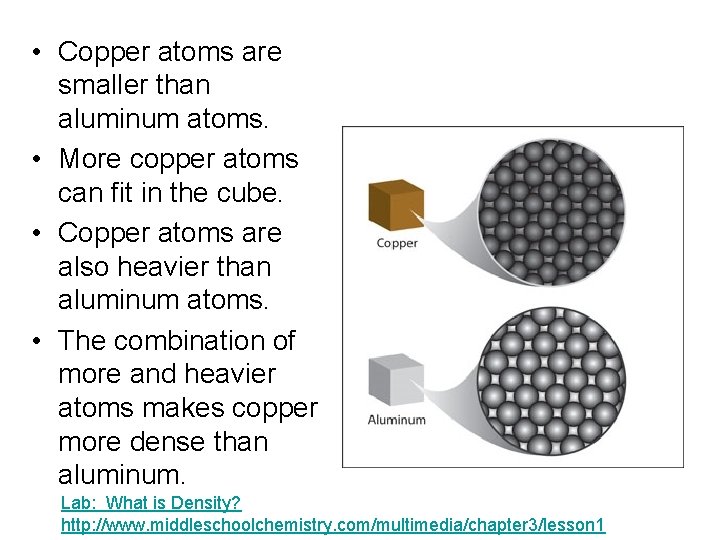
• Copper atoms are smaller than aluminum atoms. • More copper atoms can fit in the cube. • Copper atoms are also heavier than aluminum atoms. • The combination of more and heavier atoms makes copper more dense than aluminum. Lab: What is Density? http: //www. middleschoolchemistry. com/multimedia/chapter 3/lesson 1

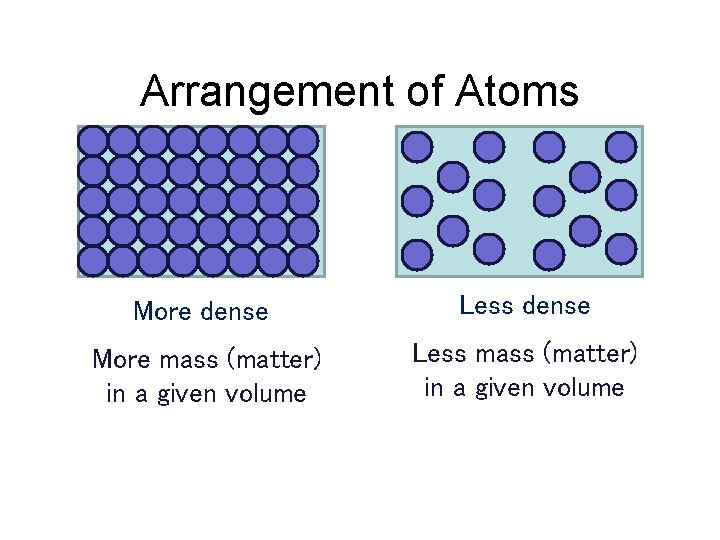
Arrangement of Atoms More dense Less dense More mass (matter) in a given volume Less mass (matter) in a given volume

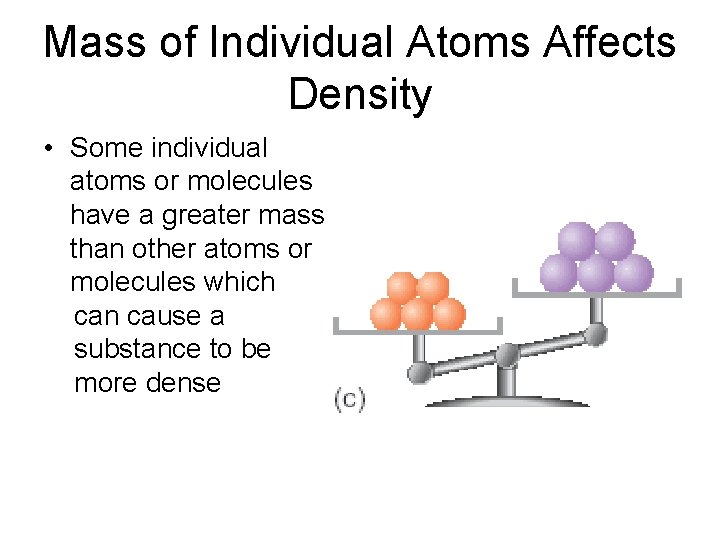
Mass of Individual Atoms Affects Density • Some individual atoms or molecules have a greater mass than other atoms or molecules which can cause a substance to be more dense


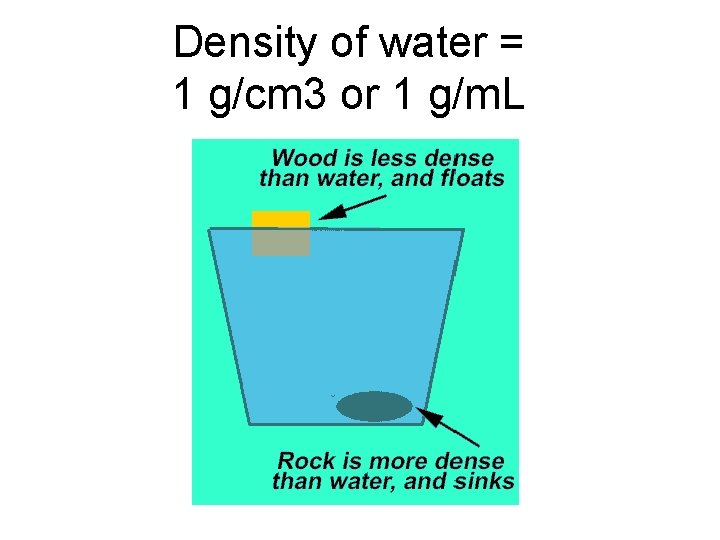
Density of water = 1 g/cm 3 or 1 g/m. L

Formula: Problem: Show Work: Answer: 2. The density of water is 1 g/cm 3. Will the ice cube sink or float in water?

Ice is less dense than water


Seven Layer Density Column Material. Density in g/cm 3 or g/m. L Rubbing Alcohol. 79 g/cm 3 Lamp Oil. 80 g/cm 3 Baby Oil. 83 g/cm 3 Vegetable Oil. 92 g/cm 3 Ice. Cube. 92 g/cm 3 Water 1. 00 g/cm 3 Milk 1. 03 g/cm 3 Dawn Dish Soap 1. 06 g/cm 3 Light Corn Syrup 1. 33 g/cm 3 Maple Syrup 1. 37 g/cm 3 Honey 1. 42 g/cm 3

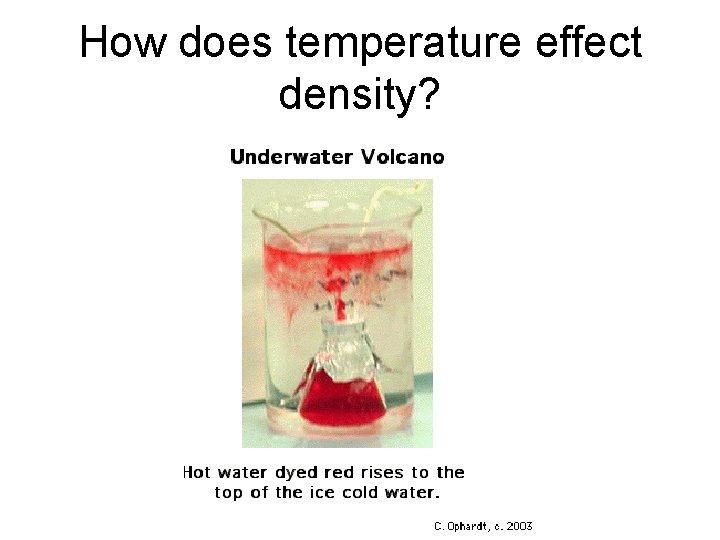
How does temperature effect density?

DMV Triangle m is the top of the mountain

Warm Up 1. A block of aluminum occupies a volume of 15. 0 m. L and weighs 40. 5 g. What is its density? 2. Mercury metal is poured into a graduated cylinder that holds exactly 22. 5 m. L. The mercury used to fill the cylinder weighs 306 g. Calculate the density of the mercury. Use this format for both problems: Formula: Problem: Show Work: Density =

Warm Up 1. A rectangular solid of unknown density is 5 meters long, 2 meters high and 4 meters wide. The mass of this solid is 300 grams. What is its density? Formula: Problem: Show Work: Volume = Density =

Warm Up 1. The density of lead is 11 g/m. L. What would the volume of a 200. 0 g sample of this metal be? Formula: Problem: Show Work: Density =

Volume Challenge #1 R. Bryant 2008 WSMS – Based on “Science Starters” by T. Trimpe at http: //sciencespot. net

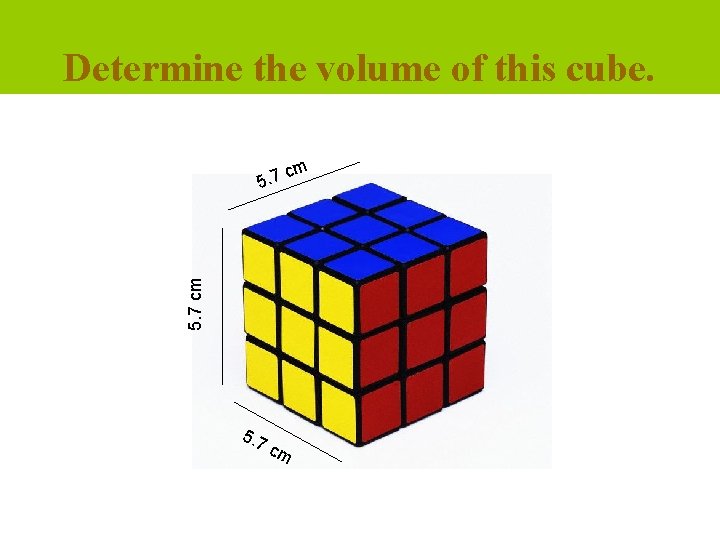
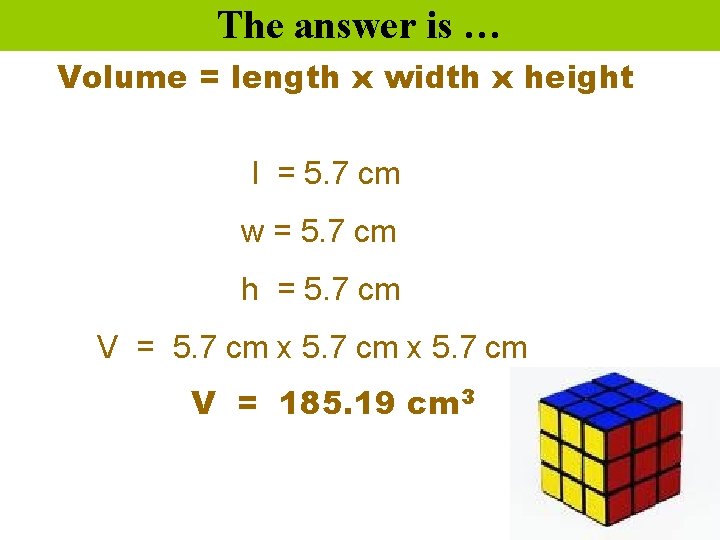
Determine the volume of this cube. m 5. 7 cm c 5. 7 cm

The answer is … Volume = length x width x height l = 5. 7 cm w = 5. 7 cm h = 5. 7 cm V = 5. 7 cm x 5. 7 cm V = 185. 19 cm 3

Density #1 R. Bryant 2008 WSMS – Based on “Science Starters” by T. Trimpe at http: //sciencespot. net

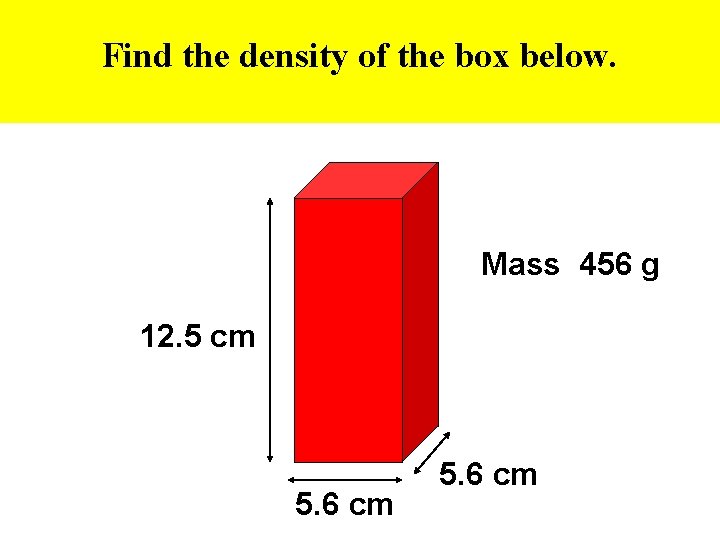
Find the density of the box below. Mass 456 g 12. 5 cm 5. 6 cm

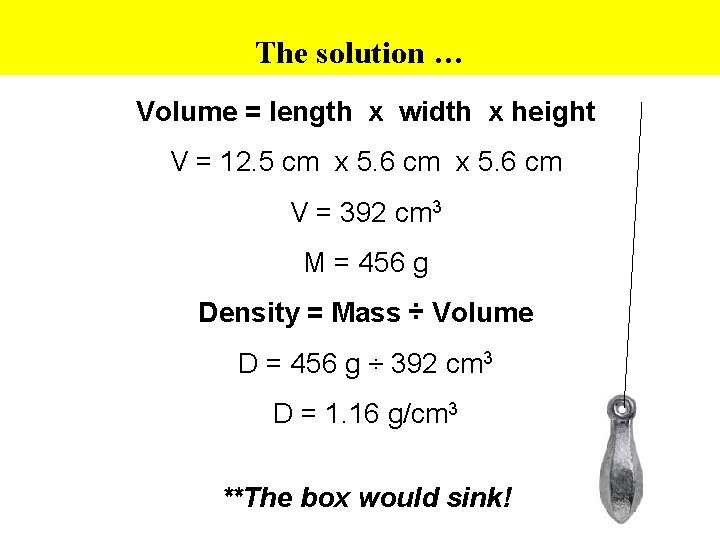
The solution … Volume = length x width x height V = 12. 5 cm x 5. 6 cm V = 392 cm 3 M = 456 g Density = Mass ÷ Volume D = 456 g ÷ 392 cm 3 D = 1. 16 g/cm 3 **The box would sink!

Density #2 R. Bryant 2008 WSMS – Based on “Science Starters” by T. Trimpe at http: //sciencespot. net

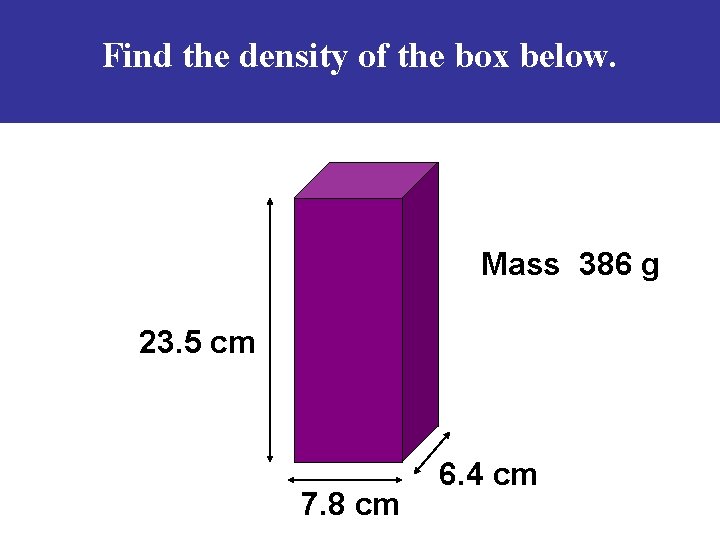
Find the density of the box below. Mass 386 g 23. 5 cm 7. 8 cm 6. 4 cm

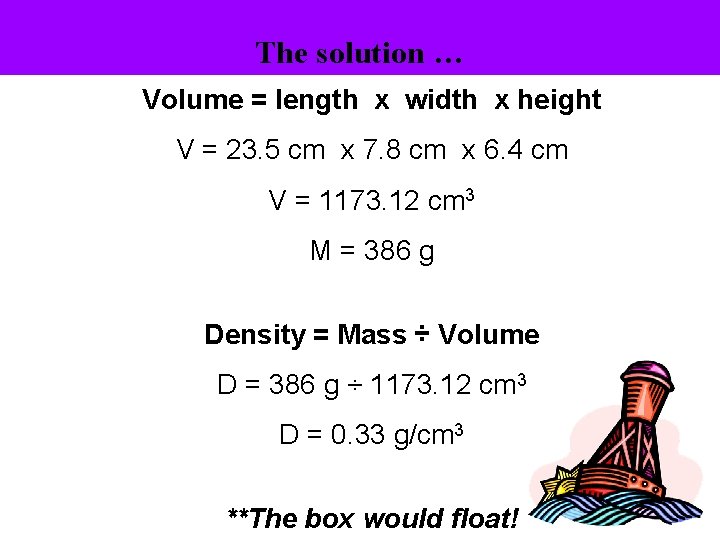
The solution … Volume = length x width x height V = 23. 5 cm x 7. 8 cm x 6. 4 cm V = 1173. 12 cm 3 M = 386 g Density = Mass ÷ Volume D = 386 g ÷ 1173. 12 cm 3 D = 0. 33 g/cm 3 **The box would float!

Density #3 R. Bryant 2008 WSMS – Based on “Science Starters” by T. Trimpe at http: //sciencespot. net

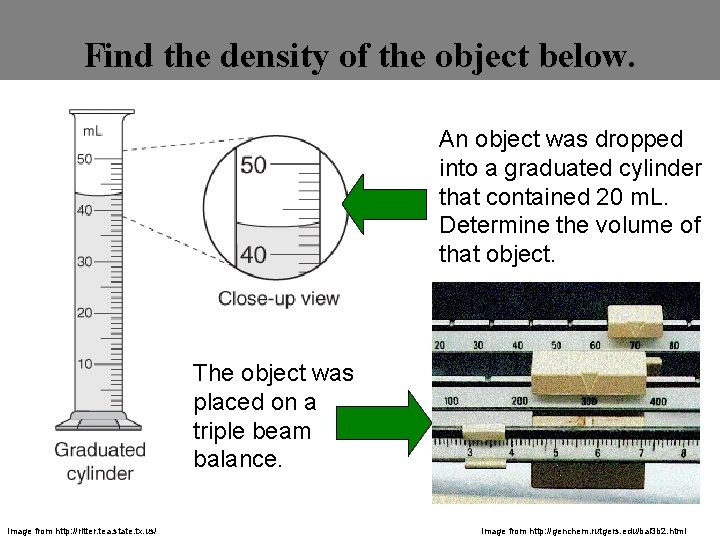
Find the density of the object below. An object was dropped into a graduated cylinder that contained 20 m. L. Determine the volume of that object. The object was The mass placed on a triple beam balance. Image from http: //ritter. tea. state. tx. us/ Image from http: //genchem. rutgers. edu/bal 3 b 2. html

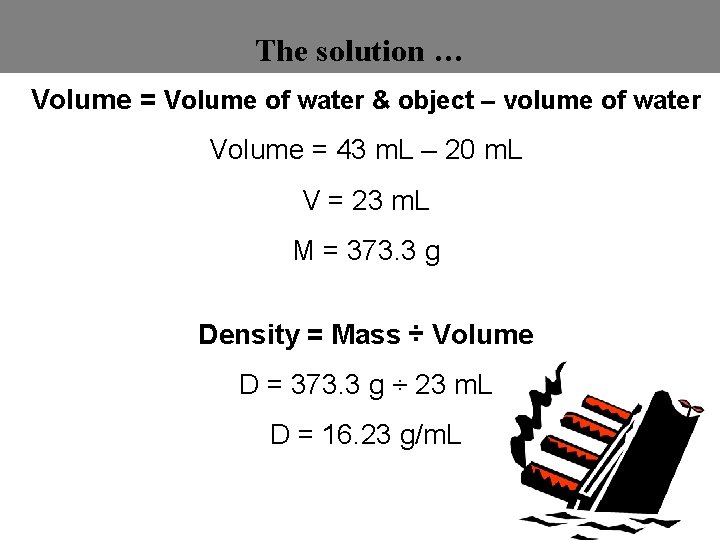
The solution … Volume = Volume of water & object – volume of water Volume = 43 m. L – 20 m. L V = 23 m. L M = 373. 3 g Density = Mass ÷ Volume D = 373. 3 g ÷ 23 m. L D = 16. 23 g/m. L

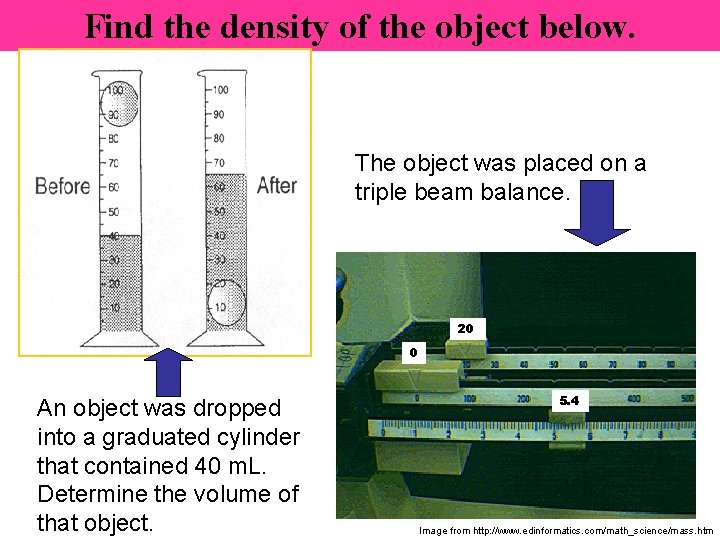
Density #4 R. Bryant 2008 WSMS – Based on “Science Starters” by T. Trimpe at http: //sciencespot. net

Find the density of the object below. The object was placed on a triple beam balance. 20 0 An object was dropped into a graduated cylinder that contained 40 m. L. Determine the volume of that object. 5. 4 Image from http: //www. edinformatics. com/math_science/mass. htm

The solution … Volume = Volume of water & object – volume of water V = 65 m. L – 40 m. L V = 25 m. L M = 25. 4 g Density = Mass ÷ Volume D = 25. 4 g ÷ 25 m. L D = 1. 01 g/m. L ** The object will sink!

• Eureka video on volume and density: • http: //www. youtube. com/watch? v=GMNp. P g. LT 8 Fk

• Eureka Video on volume and density http: //www. youtube. com/watch? v=GMNp. Pg LT 8 Fk • Eureka Video on density and buoyancy • Dr Carlson on density (displacement) • http: //www. youtube. com/watch? v=14 nah. P _FVn. M