DENSITY Big Daddy D 1 Definition of Density
DENSITY… Big Daddy “D”
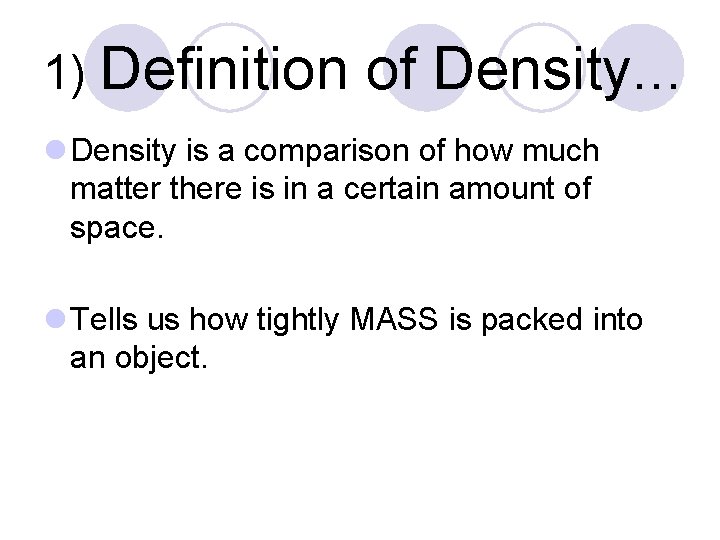
1) Definition of Density. . . l Density is a comparison of how much matter there is in a certain amount of space. l Tells us how tightly MASS is packed into an object.
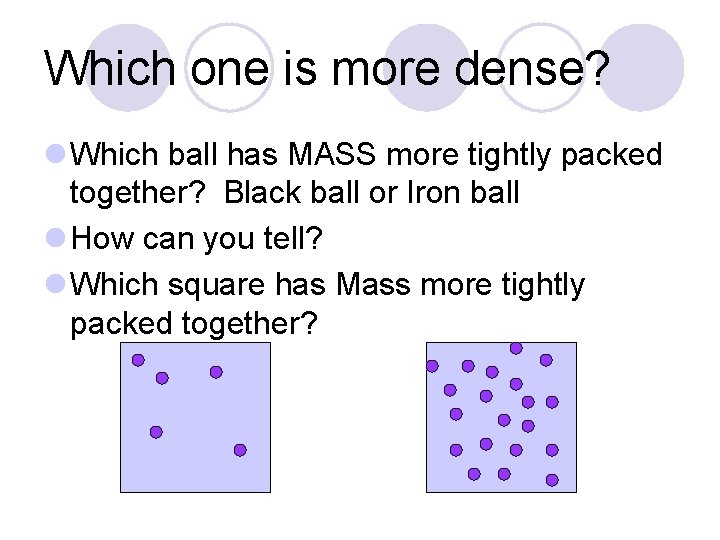
Which one is more dense? l Which ball has MASS more tightly packed together? Black ball or Iron ball l How can you tell? l Which square has Mass more tightly packed together?

Which one is more dense? l Acitivty: Students in Classroom OR Students in Hallway? ? ? Hallway Çlassroom
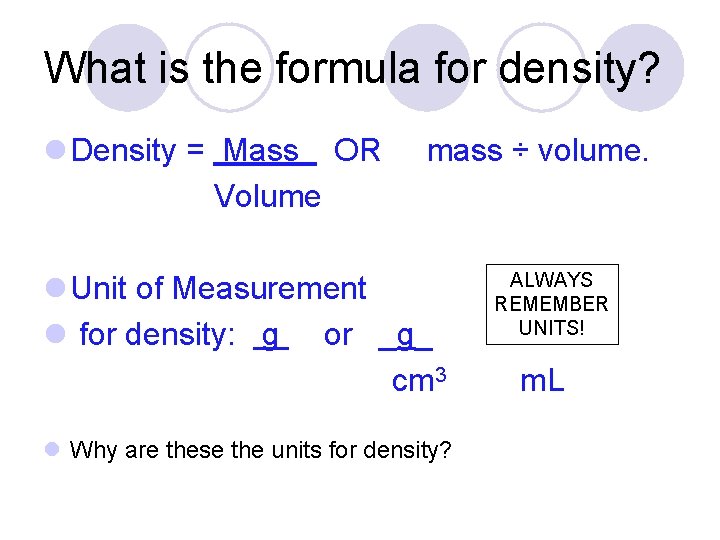
What is the formula for density? l Density = Mass OR Volume mass ÷ volume. l Unit of Measurement l for density: g or _g_ cm 3 l Why are these the units for density? ALWAYS REMEMBER UNITS! m. L

3) Tricks to remember Density… l Density is a relationship between MASS & VOLUME. l Mass and Volume are in LOVE. l The symbol for LOVE is a l What letter does the top half of the heart look like? M the bottom half? V
Tricks to remember Density… l Mountains are over Valleys

4) Important things to remember… l 1 m. L = 1 cm 3 l Unit of measurement for Density of a LIQUID is g/m. L l Unit of measurement for Density of a SOLID is g/cm 3 l Density of WATER = 1 g/m. L l If you have 10 grams of water, how many m. L?
5) Relationship of Density… l The relationship is MASS VOLUME OR Mass DIVIDED by Volume *This relationship tells us how tightly mass is packed into an object.

Solving problems using DENSITY….

Let’s try a density problem together l Frank has a paper clip. It has a mass of 9 g and a volume of 3 cm 3. What is its density? l Frank also has an eraser. It has a mass of 3 g, and a volume of 1 cm 3. What is its density?

Work on these problems with your neighbor. l Jack has a rock. The rock has a mass of 6 g and a volume of 3 cm 3. What is the density of the rock? l Jill has a gel pen. The gel pen has a mass of 8 g and a volume of 2 cm 3. What is the density of the gel pen?

Now, try these on your own. l Ima Fraid has a watch. It has a mass of 4 g and a volume of 2 cm 3. What is the density of the watch? l Cat Woman has a wallet. It has a mass of 15 g and a volume of 5 cm 3. What is the density of the wallet?

3 Density Rules l Rule #1 When mass stays the same but volume increases, Density does the opposite at the volume.

3 Density Rules l Rule #2 When volume stays the same but Mass changes, Density copies the mass,

3 Density RULES. . l Rule #3 When BOTH mass and volume increase or BOTH decrease, density stays the same!

Liquid Layers l If you pour together liquids that don’t mix and have different densities, they will form liquid layers. l The liquid with the highest density will be on the bottom. l The liquid with the lowest density will be on the top.
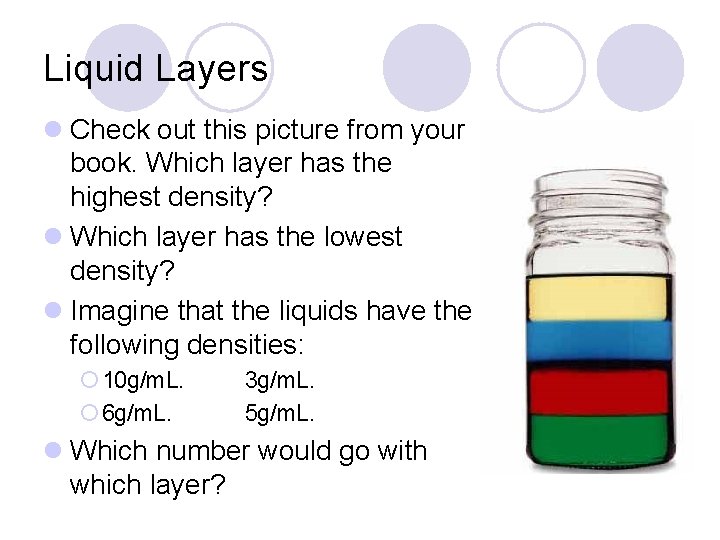
Liquid Layers l Check out this picture from your book. Which layer has the highest density? l Which layer has the lowest density? l Imagine that the liquids have the following densities: ¡ 10 g/m. L. ¡ 6 g/m. L. 3 g/m. L. 5 g/m. L. l Which number would go with which layer?
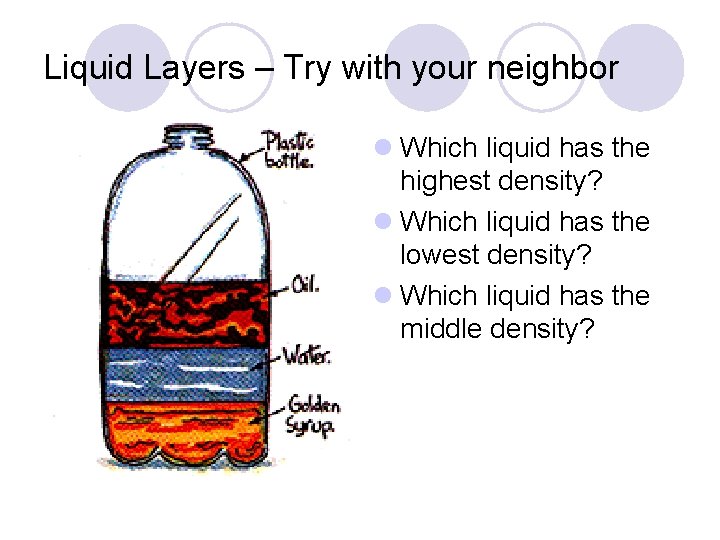
Liquid Layers – Try with your neighbor l Which liquid has the highest density? l Which liquid has the lowest density? l Which liquid has the middle density?

Liquid Layers – Try on your own! l Imagine that the liquids on the right have the following densities: ¡ 15 g/m. L ¡ 3 g/m. L ¡ 7 g/m. L 10 g/m. L 9 g/m. L 12 g/m. L l Match the colors to the correct densities. 3 g/m. L 7 g/m. L 9 g/m. L 10 g/m. L 12 g/m. L 15 g/m. L

Review l What is the formula for density? l What happens if you pour together liquids that have different densities? l Will the liquid on the top have the highest or lowest density? l Will the liquid on the bottom have the highest or lowest density?

Super Scientist Question of the Day l Jake has a book, a ruler, and a balance. l How can Jake find the density of the book with the tools he has?
- Slides: 22