Introduction to Earth Science Prologue Part II Properties





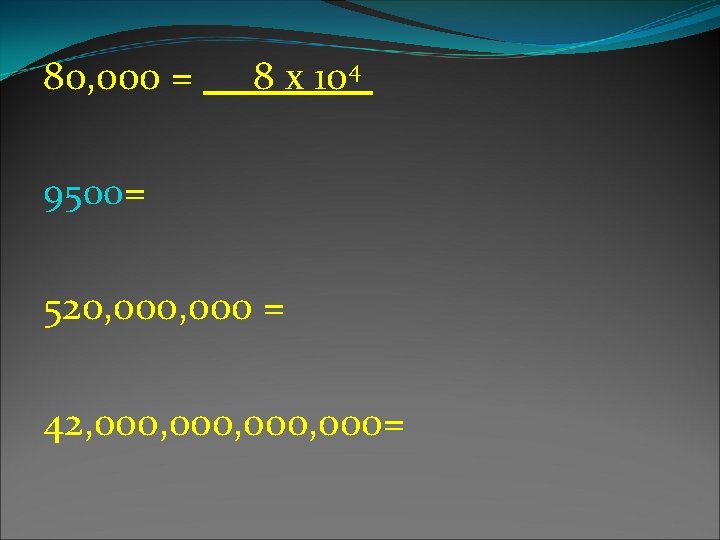













- Slides: 33

Introduction to Earth Science Prologue – Part II Properties of the Environment Measurements Percentage Deviation from accepted value Density

Metric A way of describing observation using numbers. q. All measurements contains one of the three dimensional quantities - Mass , Time or Length q. Both the quantity and the number are need for the number to be understood. q. To know worldwide metric system - The International System of Units ( SI) and the English System. q

Metric Measurements

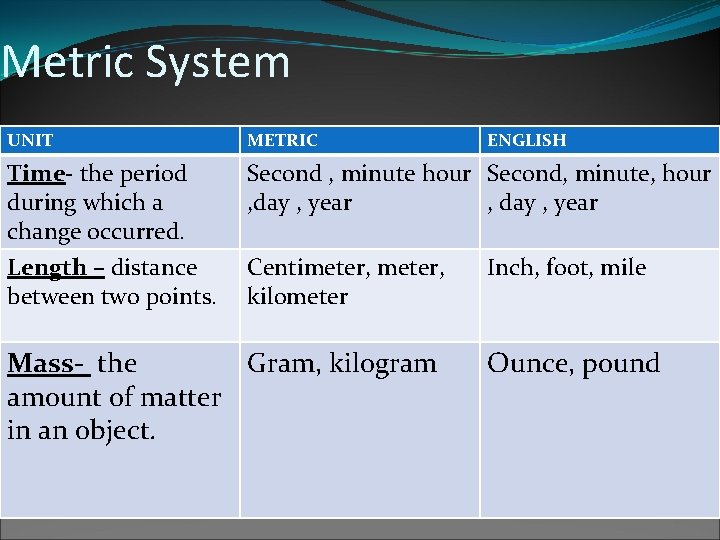
Metric System UNIT METRIC Time- the period during which a change occurred. Length – distance between two points. Second , minute hour Second, minute, hour , day , year Centimeter, kilometer Mass- the Gram, kilogram amount of matter in an object. ENGLISH Inch, foot, mile Ounce, pound

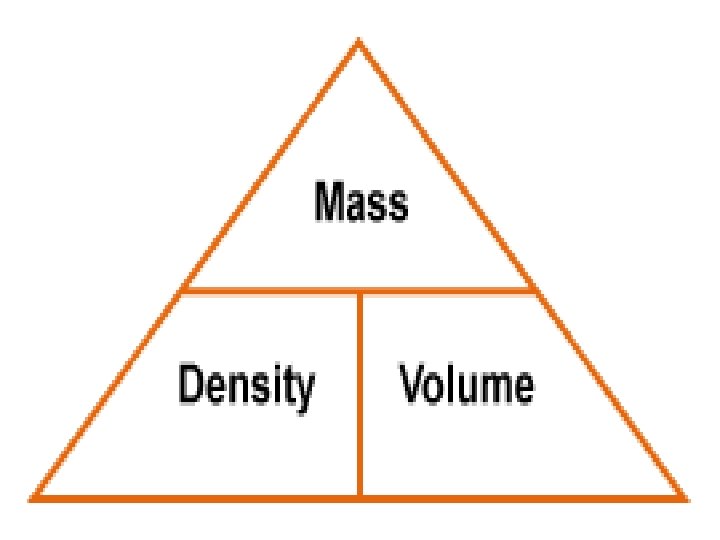
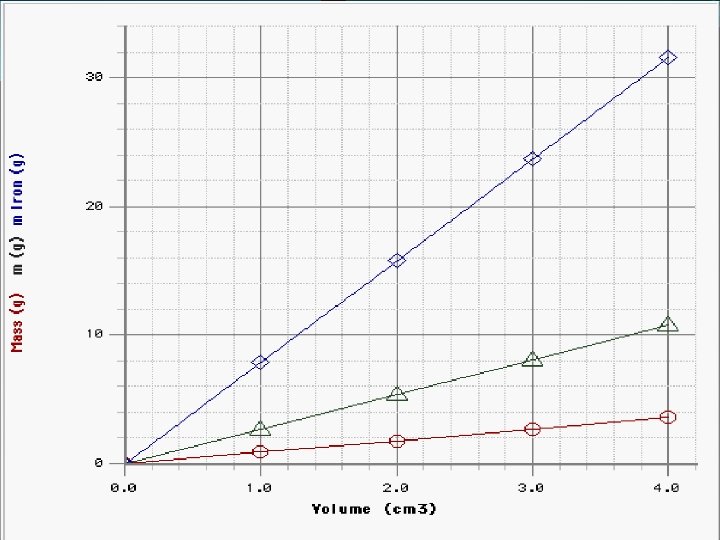
Volume = length x width x Other Properties of height or V= lvh Mater Density = mass/ volume q. Some properties of matter are best or d= m / v described using combinations. Units ? - grams / cubic centimeter or q. Volume q. Density q. Pressure q. Speed g/cm³ -> for solids grams/ milliliter o g/ml - or liquids

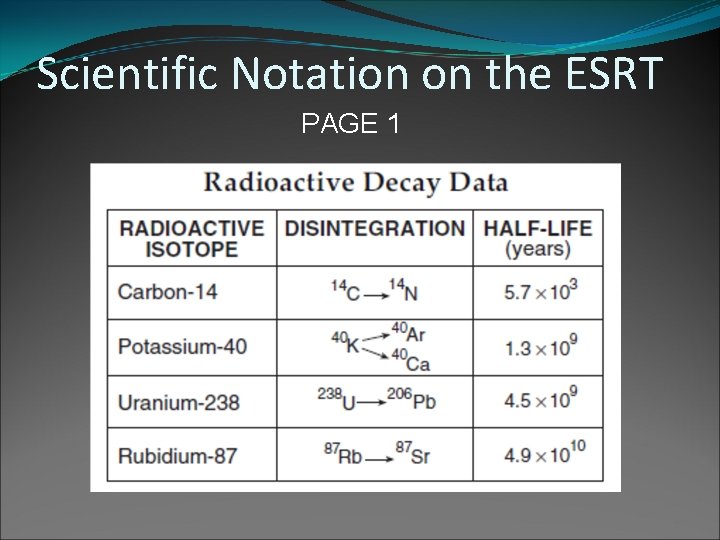
Scientific Notation: A number written as the product of a coefficient between 1 and 10 and a power of 10. U 238 half life = 4. 5 x 109 years 4, 500, 000 years Diameter of an atom = 2. 44 x 10 -10 m 0. 0000024 m

Practice: 3. 5 x 103 = _______ 8. 6 x 105 =________ 7. 4 x 102 =________
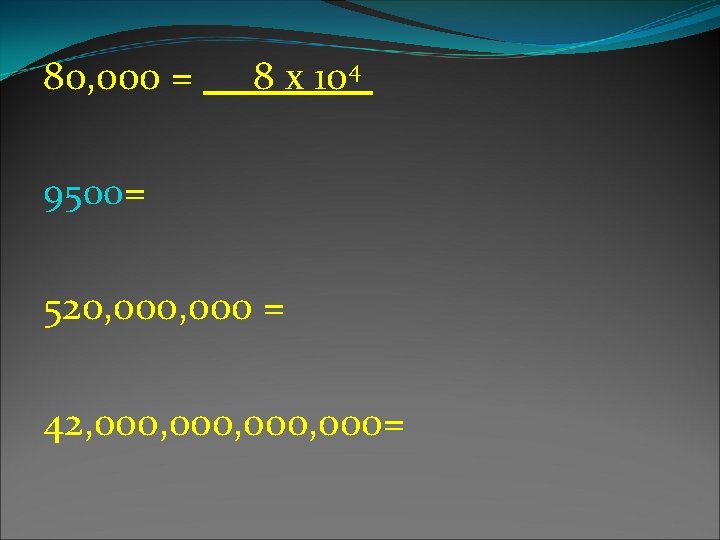
80, 000 = 8 x 104 9500= 520, 000 = 42, 000, 000=

Age of Earth 4, 600, 000 years Step 1: Write this number in scientific notation: 4. 6 x 109 years Step 2: Write this in # of years (text): 4. 6 Billion Years

Scientific Notation on the ESRT PAGE 1

? PERCENT ERROR-how wrong you are Accepted value = correct answer Measured value = your guess Temperature? Accepted value - measured value PCT ERROR = ----------------------- x 100% accepted value

Practice: A student measures a table to be 1. 9 m long. In reality it is 2. 0 m long. What is the percent error of the student? 2. 0 – 1. 9 X 100 = 5% 2. 0 A student measures a room to be 6. 9 m. If the actual length is 7. 5 m, the student’s percent error is? 7. 5 – 6. 9 X 100 = 8% 7. 5

Practice A student determines the mass of a rock to be 196 grams, but the actual mass of the rock is 200 grams. The students approximate percent deviation (percentage of error) is (1) 1. 0% (2) 2. 0% (3) 1. 5% (4) 4. 0%

Back to Density ! Density = mass/ volume or d= m / v Units ? - grams / cubic centimeter or g/cm³ -> for solids grams/ milliliter or g/ml - for liquids


Density of a regular object Density: a mass/volume ratio that does not depend on size or shape length= 10 cm width = 2 cm Mass = 240 height = 4 cm grams Volume = ? 10 cm x 2 cm x 4 cm 3 = 80 cm Density = mass/volume Density = ? 240 g / 80 cm 3 = 3 g/cm 3

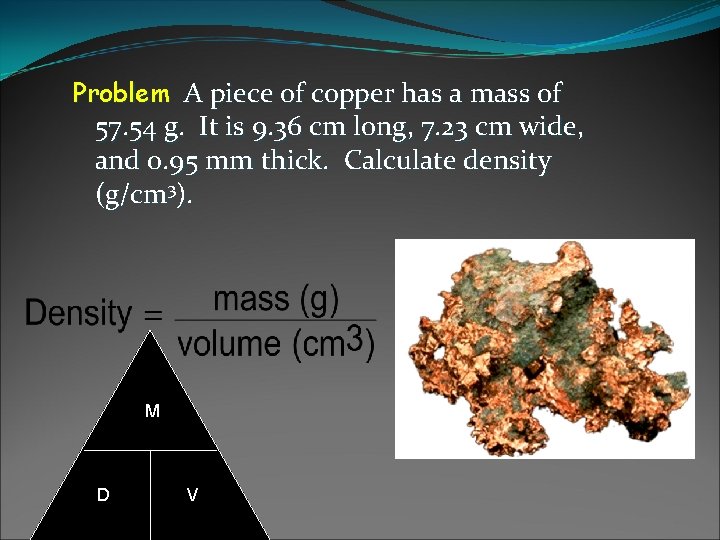
Problem A piece of copper has a mass of 57. 54 g. It is 9. 36 cm long, 7. 23 cm wide, and 0. 95 mm thick. Calculate density (g/cm 3). M D V

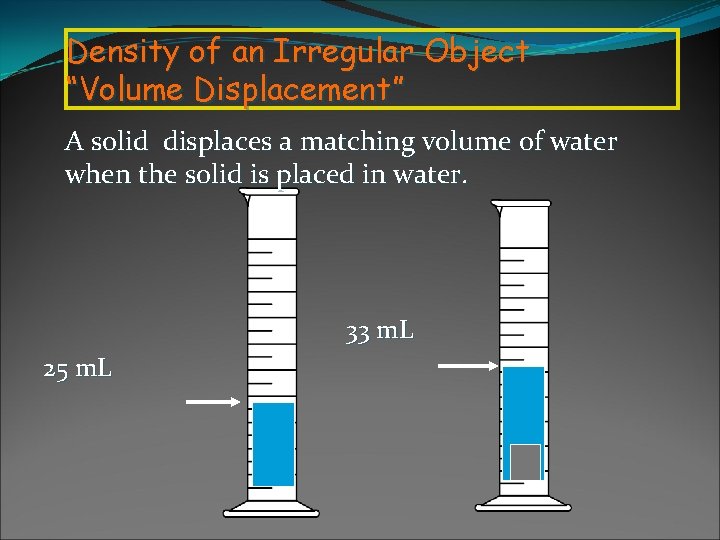
Density of an Irregular Object “Volume Displacement” A solid displaces a matching volume of water when the solid is placed in water. 33 m. L 25 m. L

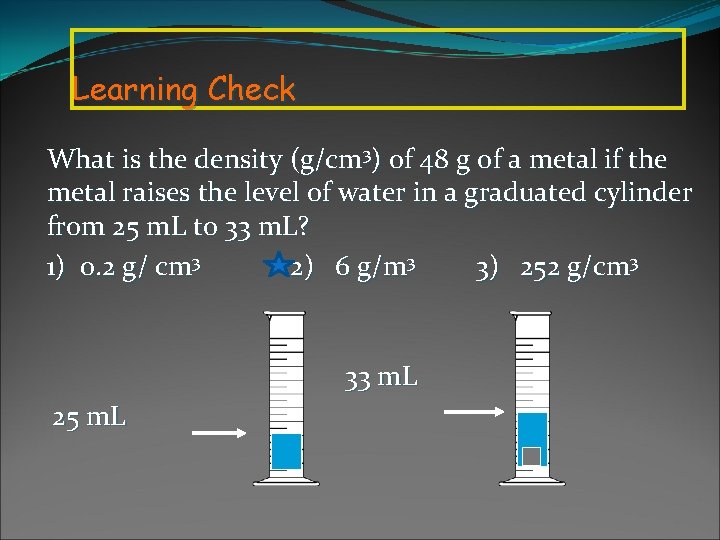
Learning Check What is the density (g/cm 3) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 m. L to 33 m. L? 1) 0. 2 g/ cm 3 2) 6 g/m 3 3) 252 g/cm 3 33 m. L 25 m. L

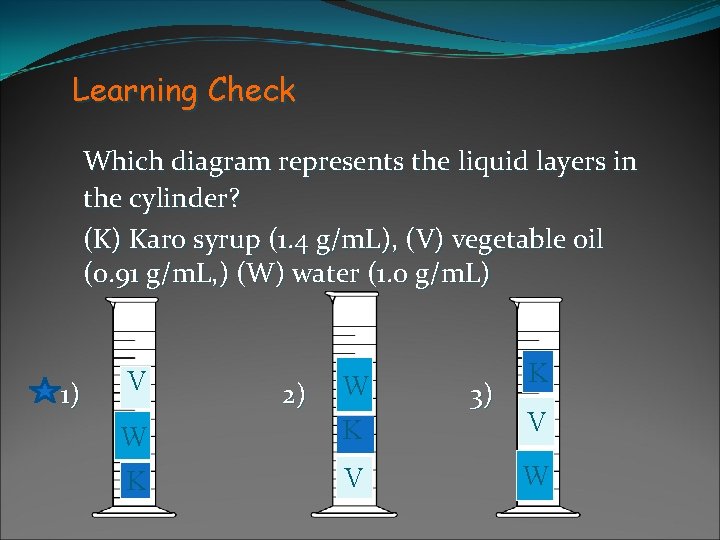
Learning Check Which diagram represents the liquid layers in the cylinder? (K) Karo syrup (1. 4 g/m. L), (V) vegetable oil (0. 91 g/m. L, ) (W) water (1. 0 g/m. L) 1) V 2) W 3) K W K V W

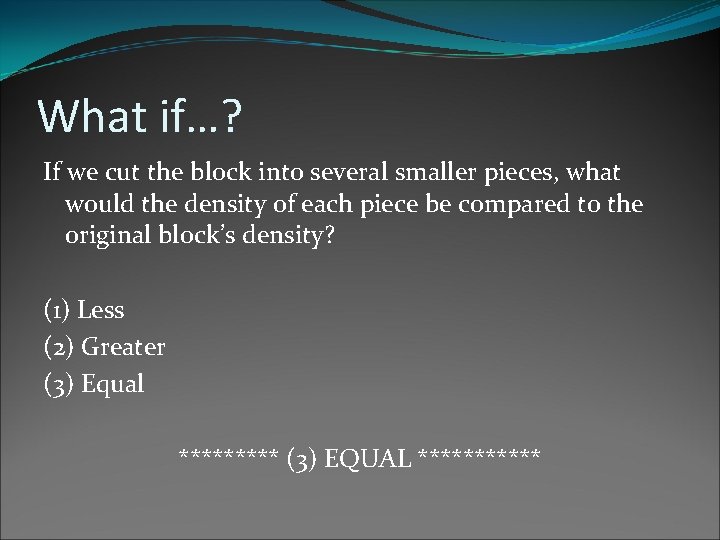
What if…? If we cut the block into several smaller pieces, what would the density of each piece be compared to the original block’s density? (1) Less (2) Greater (3) Equal ***** (3) EQUAL ******

Density, Temperature, and Volume What happens to the air inside the balloon as we heat it? What happens to the volume? What happens to the density? What if we cool the air? Draw a graph that represents this relationship.

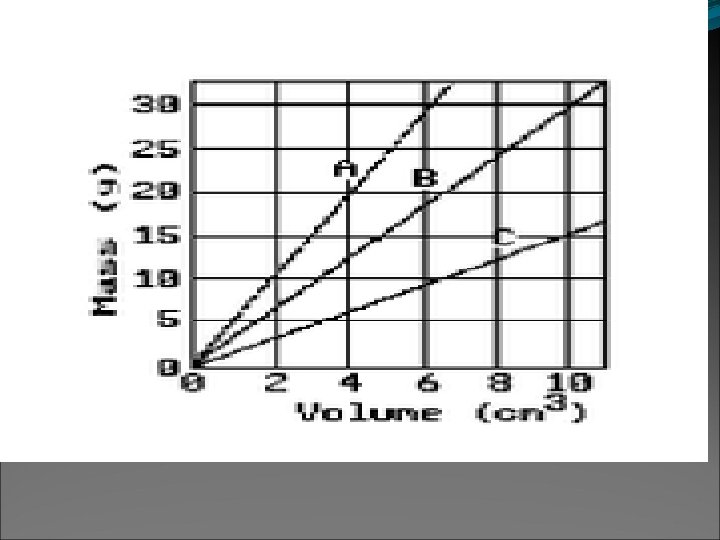
More Density Notes: 240 grams What happens to the density of this box if you increase the size/volume from 80 cm 3 to 100 cm 3? If you increase the size of the box/the volume increases (and mass stays the same) density decreases. You are dividing the mass by a larger number, thus density will decrease. Ex. 240 grams/100 cm 3 = 2. 4 g/cm 3

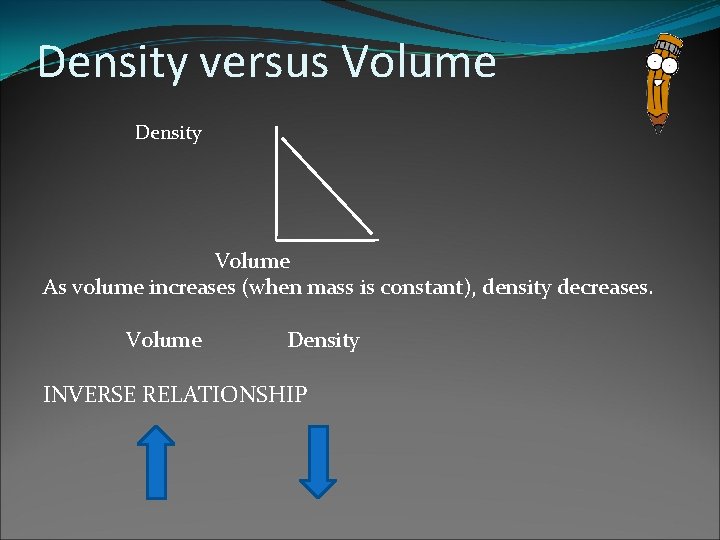
Density versus Volume Density Volume As volume increases (when mass is constant), density decreases. Volume Density INVERSE RELATIONSHIP

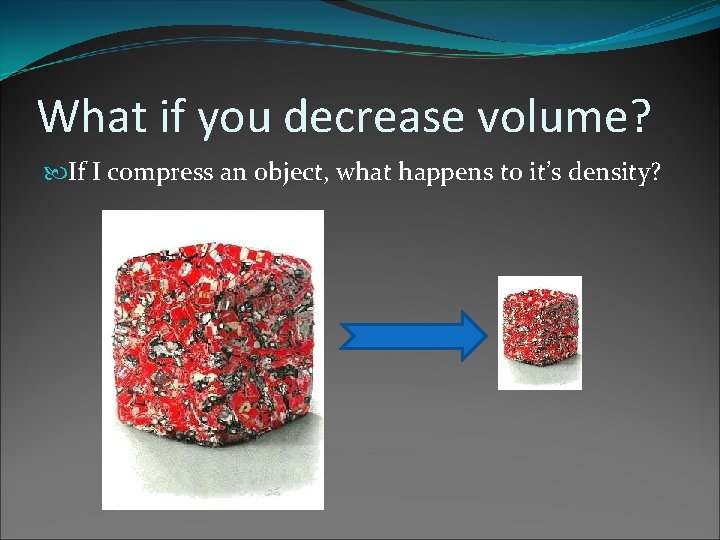
What if you decrease volume? If I compress an object, what happens to it’s density?

Which is more dense?

Which is more dense?

Which is more dense?

Which is more dense?

Average densities: -water = 1 g/cm 3 at 4 o C *** below 4 o. C the density of water decreases. Above 4 o. C, the density of water increases -Ice 0. 5 g/cm 3 -Earth = 5. 5 g/cm 3 -Saturn = 0. 7 g/cm 3 -human = ?

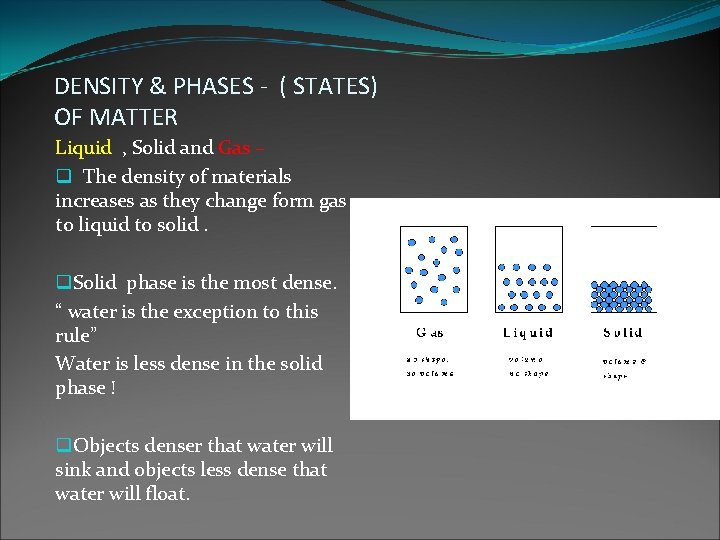
DENSITY & PHASES - ( STATES) OF MATTER Liquid , Solid and Gas – q The density of materials increases as they change form gas to liquid to solid. q. Solid phase is the most dense. “ water is the exception to this rule” Water is less dense in the solid phase ! q. Objects denser that water will sink and objects less dense that water will float.


 Earth science prologue review
Earth science prologue review Earth science regents part d
Earth science regents part d Chapter 1 introduction to earth science
Chapter 1 introduction to earth science Earth science introduction
Earth science introduction Favourite subject science
Favourite subject science Science fusion think central
Science fusion think central Inner core physical properties
Inner core physical properties Families of elements
Families of elements Earth mantle definition
Earth mantle definition Which part of earth contains most of its mass
Which part of earth contains most of its mass Which part of the earth is the hottest?
Which part of the earth is the hottest? Part of the earth
Part of the earth How many layers are there in the earth
How many layers are there in the earth Extensive properties and intensive properties
Extensive properties and intensive properties Chemical property definition
Chemical property definition 7-3 practice more multiplication properties of exponents
7-3 practice more multiplication properties of exponents 3-2 properties of parallel lines answers
3-2 properties of parallel lines answers Part whole model subtraction
Part whole model subtraction Part to part ratio definition
Part to part ratio definition Brainpop ratios
Brainpop ratios Technical description examples
Technical description examples Standard bar parts and layout
Standard bar parts and layout The part of a shadow surrounding the darkest part
The part of a shadow surrounding the darkest part Part to part variation
Part to part variation Which weather map symbol is used to represent violently
Which weather map symbol is used to represent violently Earth science sol 2010
Earth science sol 2010 Nys earth science lab practical
Nys earth science lab practical Earth science lab practical
Earth science lab practical Earth science grade 9
Earth science grade 9 Dynamic equilibrium earth science
Dynamic equilibrium earth science The hydrosphere includes
The hydrosphere includes Science jeopardy 8th grade
Science jeopardy 8th grade Geology earth science definition
Geology earth science definition Environmental science final study guide
Environmental science final study guide