U 1 D 12 DENSITY NOTES How mass












- Slides: 19

U 1 D 12 DENSITY NOTES How mass compares with volume.

U 1 D 12 BELL RINGER 9/16 - ONENOTE 1 2 3 Name as many steps in the scientific method as you can. Name one qualitative observation about the room. Name one quantitative observation about the room.

OBJECTIVES Content Objective: I can define density of an object. Language Objective: I can record important notes.

FINISH AND DEBRIEF SKITTLES LAB 1) Take 3 minutes to finish what you haven't already. These are due by Wednesday. Science Practices (Skills) Asking questions (for science) and defining problems (for engineering) Developing and using models Planning and carrying out investigations Analyzing and interpreting data 2) Discuss in your groups: How can the scientific practices help us uncover truths? Using mathematics and computational thinking Constructing explanations (for science) and designing solutions (for engineering) Engaging in argument from evidence Obtaining, evaluating, and communicating information

U 1 D 12 DENSITY NOTES How mass compares with volume.

Density is the mass of one unit of volume of a substance. How many grams is 1 m. L? How many grams is 1 cm²? Density can be calculated using :

WHAT IS THE DENSITY OF AN OBJECT WITH A MASS OF 40 GRAMS IF IT HAS A VOLUME OF 80 CM³ ? Answer : D=m/v = 40 g/80 cm³ D= 0. 5 g. cm³

WHAT IS THE DENSITY OF A PIECE OF METAL WITH A MASS OF 27 GRAMS IF IT HAS A VOLUME OF 3 CM³ ? Answer : D=m/v = 27 g/3 cm³ D= 9 g. cm³

FLOAT OR SINK? OBJECTS WITH A DENSITY LESS THAN 1 G/CM³ WILL FLOAT IN WATER. WOODS, EXCEPT A FEW LIKE EBONY, HAVE DENSITY LESS THAN 1 G/CM³, SO THEY WILL FLOAT IN WATER.

METALS HAVE DENSITIES ABOVE 2. 5 G/CM³ , USUALLY BETWEEN 7 TO 20 G/CM³ , SO THEY SINK IN WATER.

RELATIONSHIP BETWEEN MASS AND VOLUME Density= Amount of Mass for a given volume Density=Mass/Volume Density plays a major role in Earth Science Identifying layers in Earth's core Ocean layers and currents Layers in the atmosphere Images from: shonscience. com, omp. gso. uri. edu, rsd 17. org

DENSITY – A UNIQUE PROPERTY Each material has a characteristic density, so density can be used to identify a material. Material Pine Wood Water Iron Density 0. 4 1. 0 7. 8 Gold 19

SIZE DOES NOT AFFECT DENSITY. The size of an object does not change its density. Density is what the material is made of, not how big or small it is. Example: Two pieces of oak wood. Little piece Mass 20 g Volume 40 cm³ Density = 0. 5 g/ cm³ Big piece Mass 60 g Volume 120 cm³ Density = 0. 5 g/ cm³

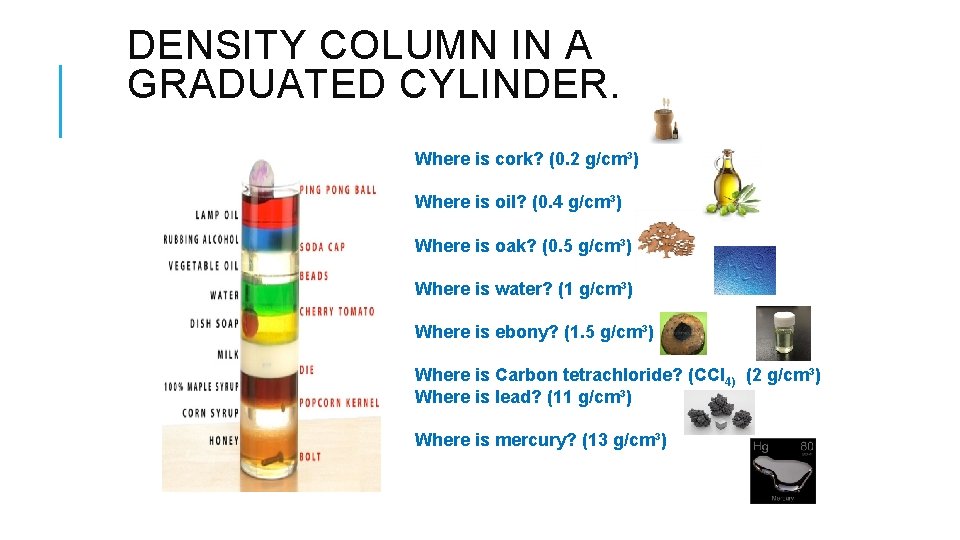
DENSITY COLUMN IN A GRADUATED CYLINDER. Where is cork? (0. 2 g/cm³) Where is oil? (0. 4 g/cm³) Where is oak? (0. 5 g/cm³) Where is water? (1 g/cm³) Where is ebony? (1. 5 g/cm³) Where is Carbon tetrachloride? (CCl 4) (2 g/cm³) Where is lead? (11 g/cm³) Where is mercury? (13 g/cm³)

QUICK WRITE – DENSITY COLUMN 1) What do you observe? 2) Why do the different substances sit in layers? Which ones sit up higher? Why?

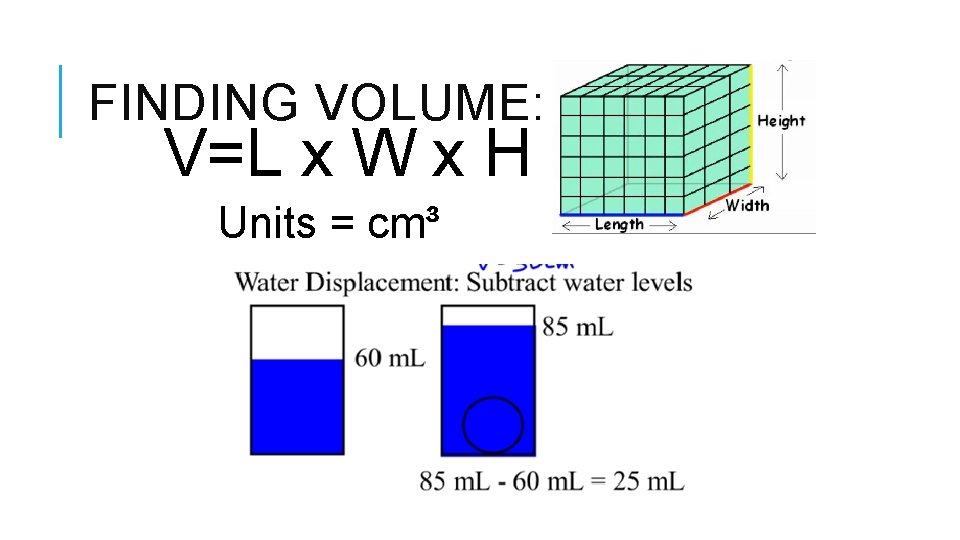
FINDING VOLUME: V=L x W x H Units = cm³

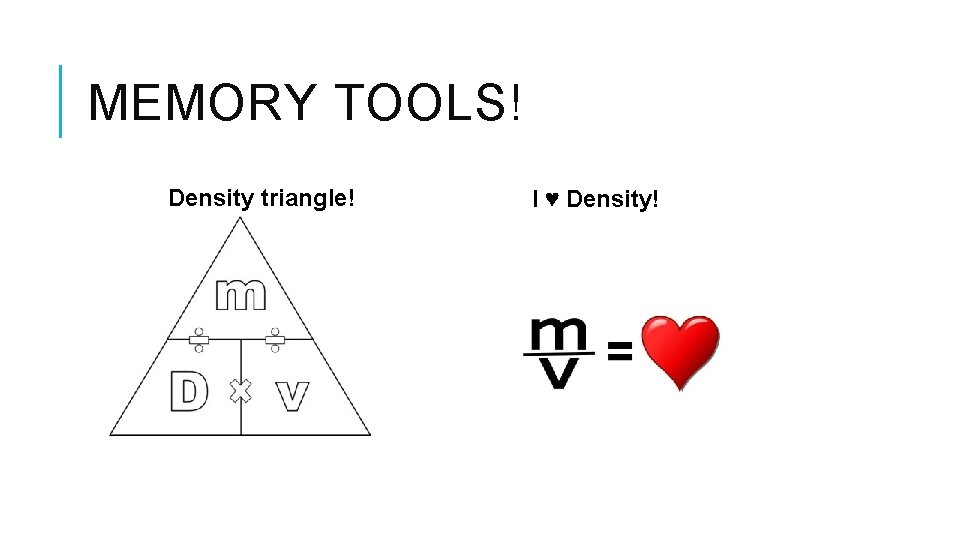
MEMORY TOOLS! Density triangle! I ♥ Density!

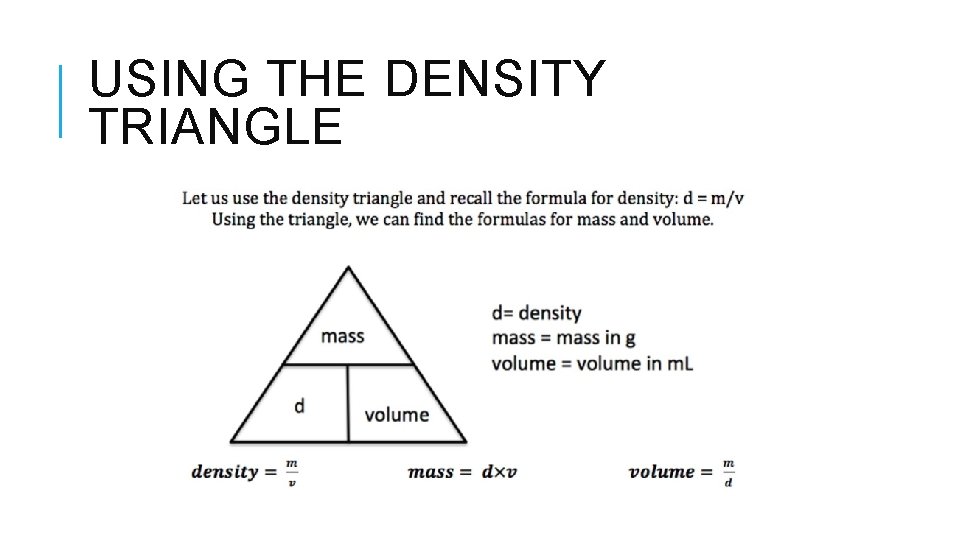
USING THE DENSITY TRIANGLE

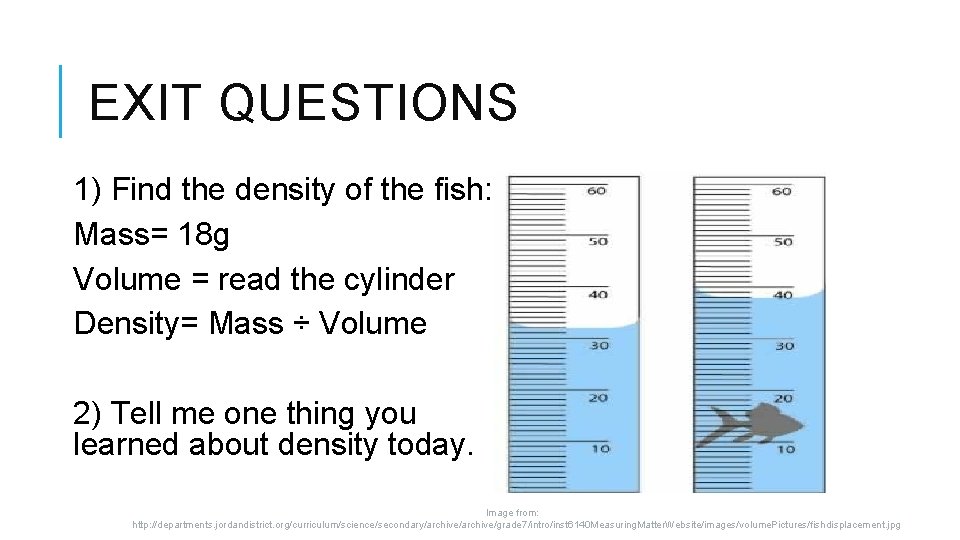
EXIT QUESTIONS 1) Find the density of the fish: Mass= 18 g Volume = read the cylinder Density= Mass ÷ Volume 2) Tell me one thing you learned about density today. Image from: http: //departments. jordandistrict. org/curriculum/science/secondary/archive/grade 7/intro/inst 6140 Measuring. Matter. Website/images/volume. Pictures/fishdisplacement. jpg