Density Equation Density D mass m volume v
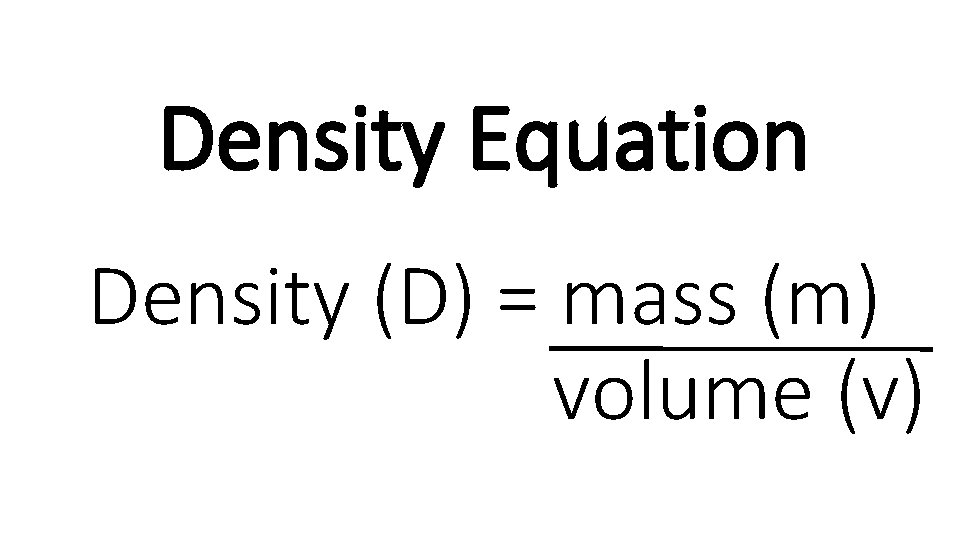





- Slides: 14
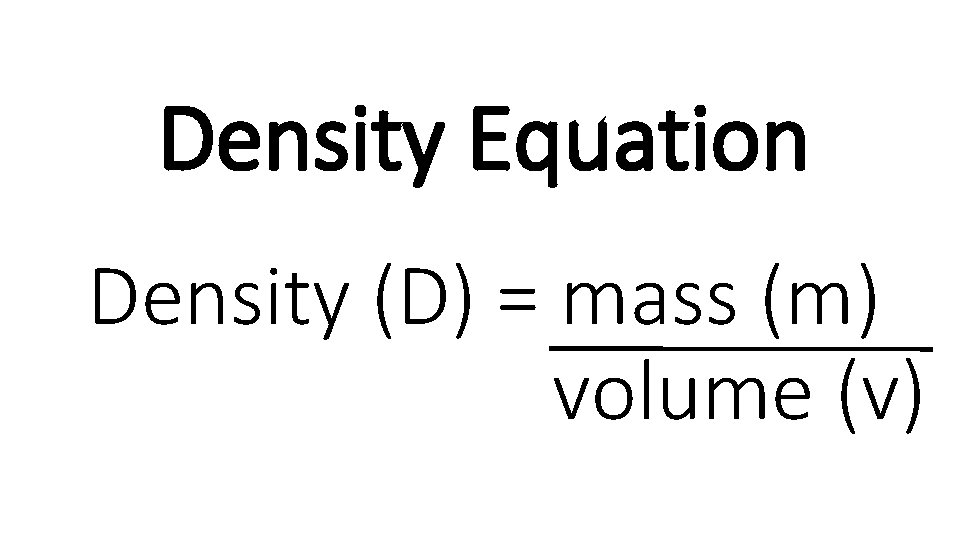
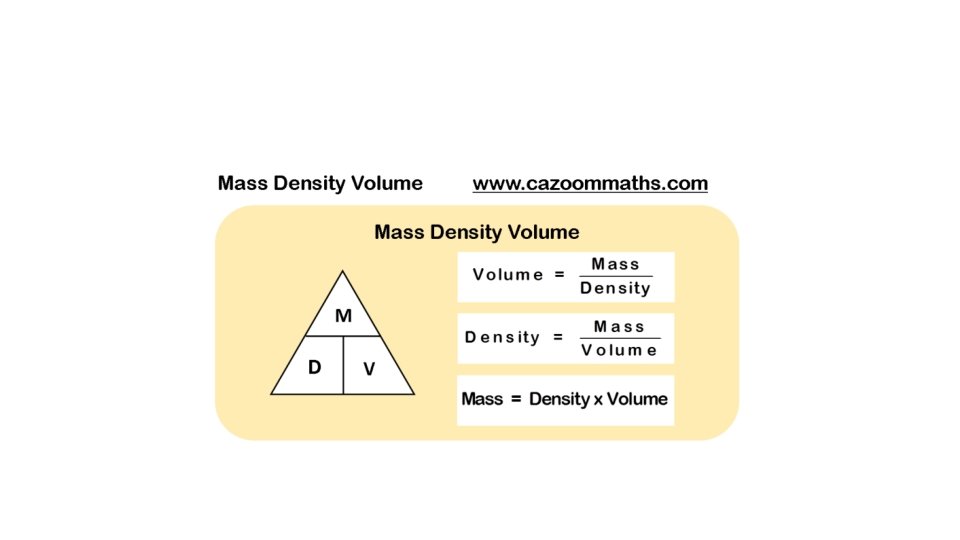
Density Equation Density (D) = mass (m) volume (v)


Using the equipment at your lab table find the density 3 (g/cm or g/m. L) of: - the block - the marble While collecting data be thinking about: - water - the hypothesis - the procedure - the IV, DV, constants, etc.

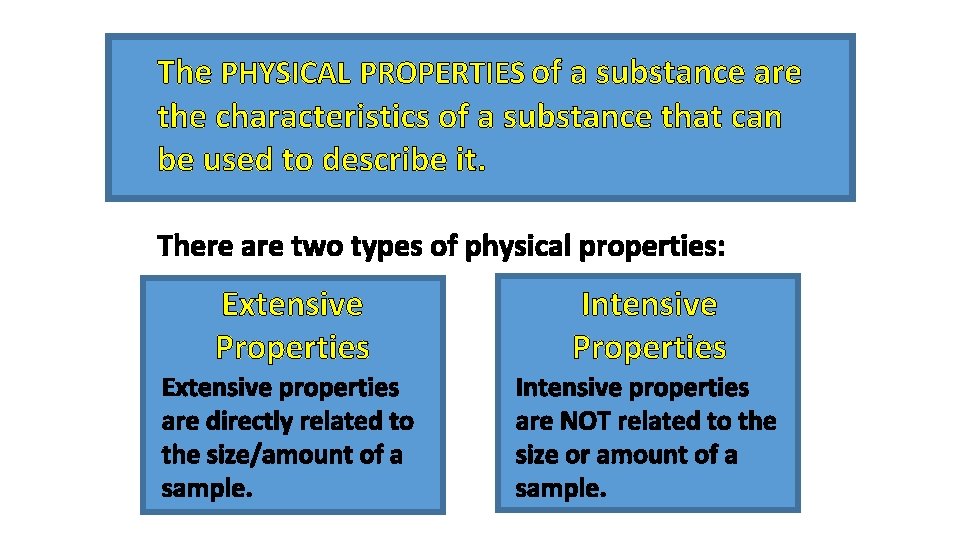
The PHYSICAL PROPERTIES of a substance are the characteristics of a substance that can be used to describe it. There are two types of physical properties: Extensive Properties Intensive Properties Extensive properties are directly related to the size/amount of a sample. Intensive properties are NOT related to the size or amount of a sample.

There are 3 primary extensive properties that are related to the size of a sample. Mass is related to the amount of matter in a substance. A larger sample has more matter, so mass is an extensive property.

Volume is the amount of space a sample occupies. A smaller sample would take up less space, so volume is an extensive property.

Length is simply how long a sample is. (Width and height are also just lengths. ) Length measures the size of a sample, so it is also an extensive property.

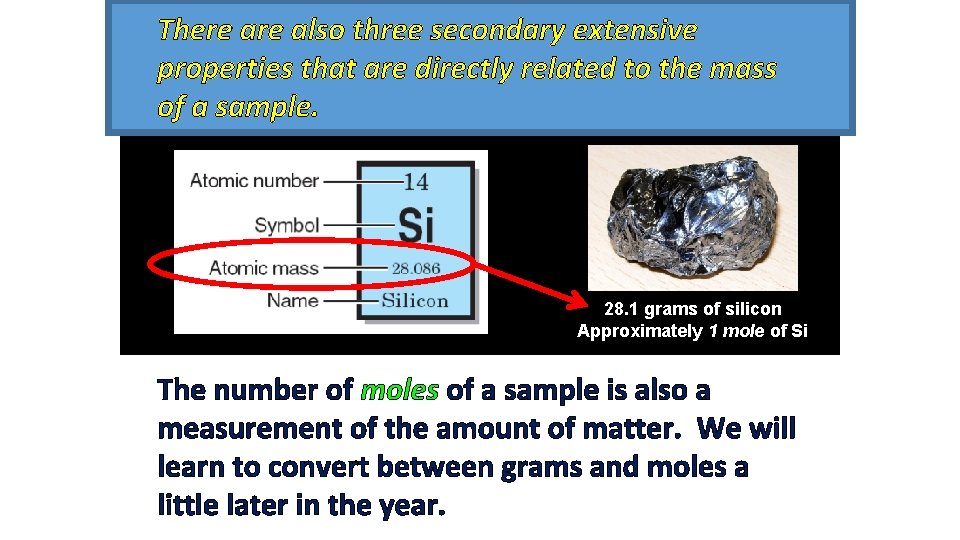
There also three secondary extensive properties that are directly related to the mass of a sample. 28. 1 grams of silicon Approximately 1 mole of Si The number of moles of a sample is also a measurement of the amount of matter. We will learn to convert between grams and moles a little later in the year.

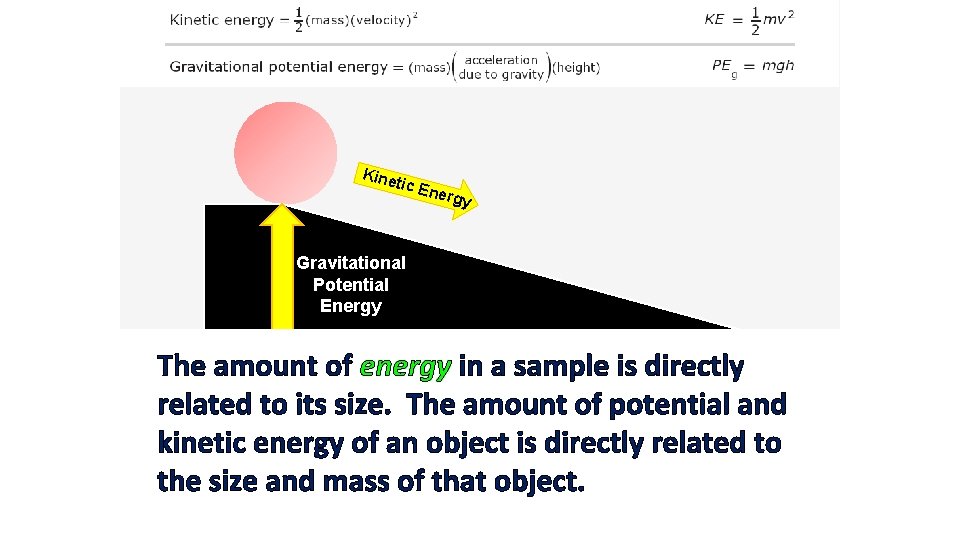
Kine tic E n ergy Gravitational Potential Energy The amount of energy in a sample is directly related to its size. The amount of potential and kinetic energy of an object is directly related to the size and mass of that object.

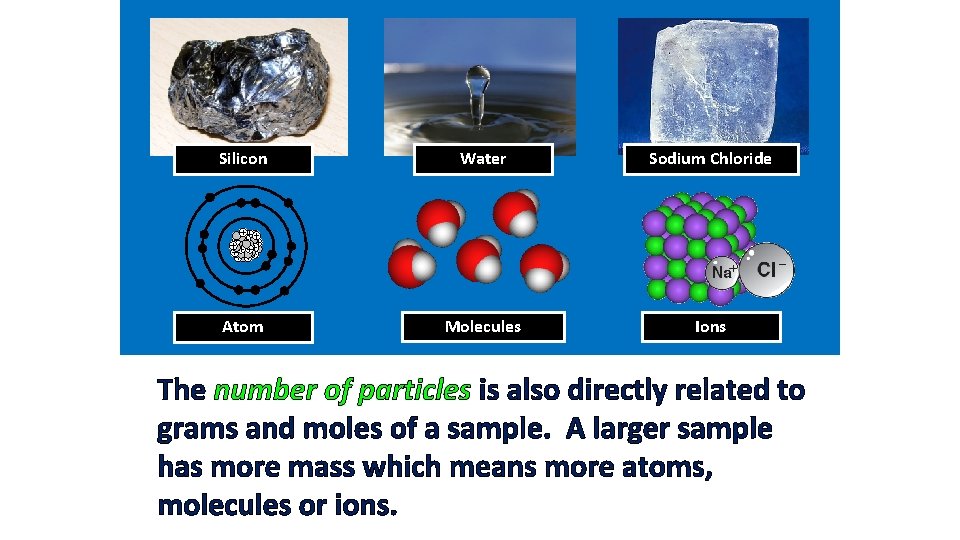
Silicon Water Sodium Chloride Atom Molecules Ions The number of particles is also directly related to grams and moles of a sample. A larger sample has more mass which means more atoms, molecules or ions.

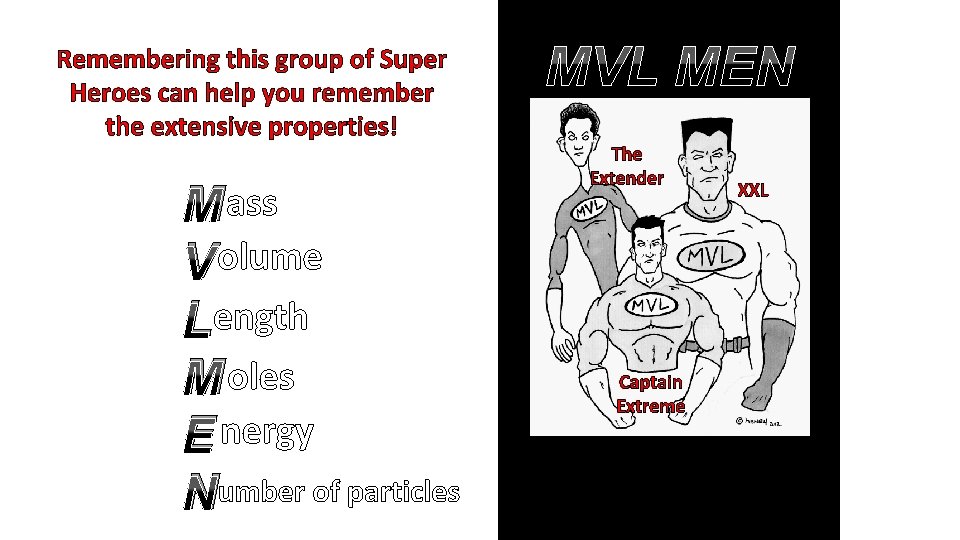
Remembering this group of Super Heroes can help you remember the extensive properties! M ass V olume Length M oles E nergy Number of particles MVL MEN The Extender XXL Captain Extreme Fighting crime one extensive property at a time!

Examples of INTENSIVE properties (not related to size): Magnetism Color State of Matter Temperature/ Boiling Point/ Melting Point

What type of physical property is density?

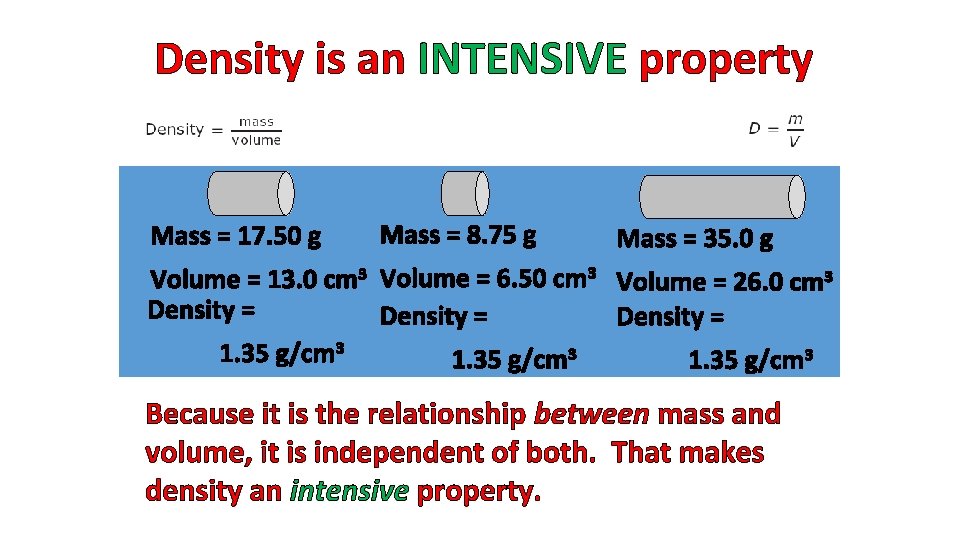
Density is an INTENSIVE property Mass = 17. 50 g Mass = 8. 75 g Mass = 35. 0 g Volume = 13. 0 cm 3 Volume = 6. 50 cm 3 Volume = 26. 0 cm 3 Density = 1. 35 g/cm 3 Because it is the relationship between mass and volume, it is independent of both. That makes density an intensive property.