Density What is Density Density is the amount








- Slides: 18

Density

What is Density? • Density is the amount of mass in a volume Or a comparison of how much matter there is in a certain amount of space. • It also determines whether something will float or sink in water • It can be calculated using the following formula: Density = Mass ÷ Volume

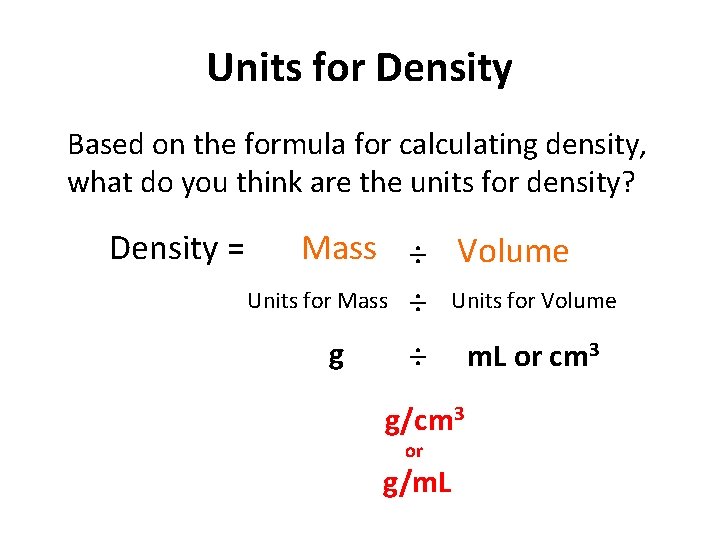
Units for Density Based on the formula for calculating density, what do you think are the units for density? Density = Mass ÷ Volume Units for Mass ÷ Units for Volume g ÷ m. L or cm 3 g/cm 3 or g/m. L

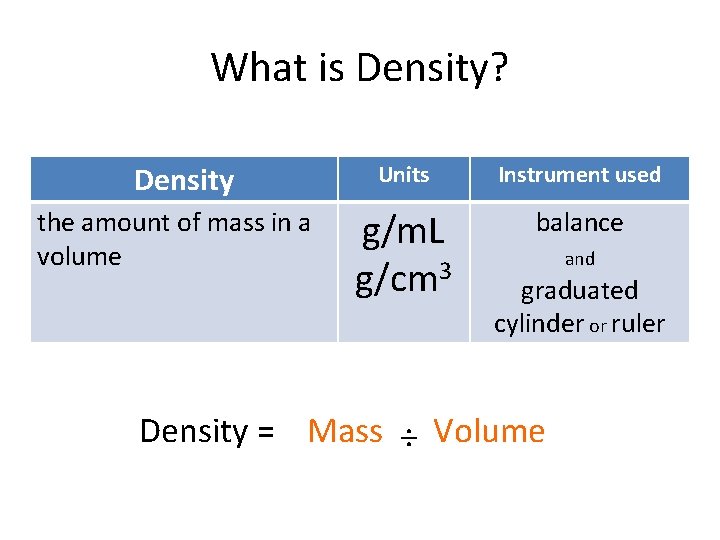
What is Density? Density the amount of mass in a volume Units Instrument used g/m. L g/cm 3 balance and graduated cylinder or ruler Density = Mass ÷ Volume

We are going to practice Density as a class. Let’s see how dense we can get! • Frank has a paper clip. It has a mass of 9 g and a volume of 3 cm 3. What is its density? Let’s do an example. • Frank also has an eraser. It has a mass of 3 g, and a volume of 1 cm 3. What is its density?

Work through these problems with a neighbor • Jack has a rock. The rock has a mass of 6 g and a volume of 3 cm 3. What is the density of the rock? Let’s do an example. • Jill has a gel pen. The gel pen has a mass of 8 g and a volume of 2 cm 3. What is the density of the gel pen?

Density = Mass ÷ Volume • Density is a physical property of matter that is always the same at a given temperature and pressure for a given substance

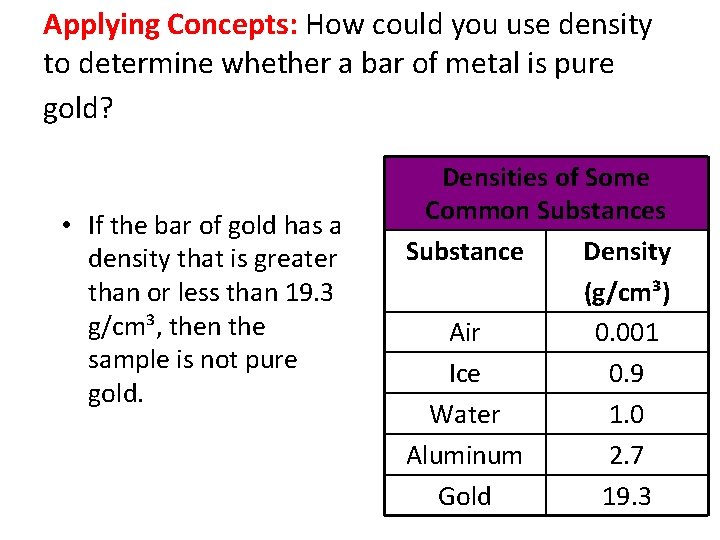
Applying Concepts: How could you use density to determine whether a bar of metal is pure gold? • If the bar of gold has a density that is greater than or less than 19. 3 g/cm³, then the sample is not pure gold. Densities of Some Common Substances Substance Density (g/cm³) Air 0. 001 Ice 0. 9 Water 1. 0 Aluminum 2. 7 Gold 19. 3

Effects of Mass and Volume on Density

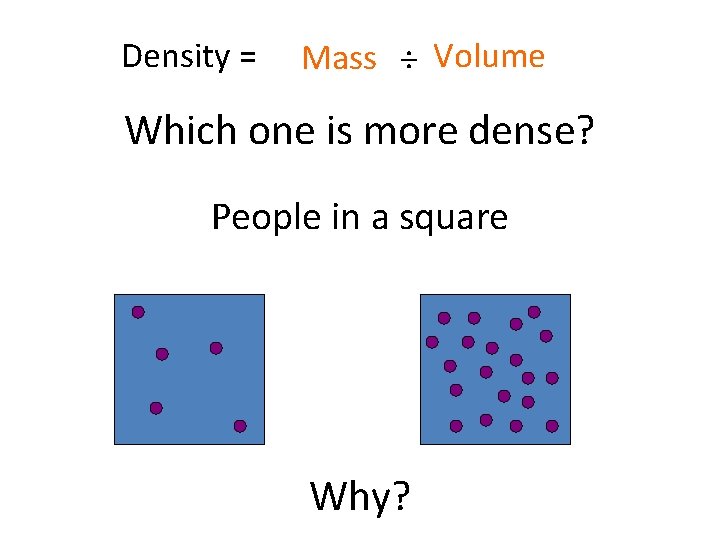
Density = Mass ÷ Volume Which one is more dense? People in a square Why?

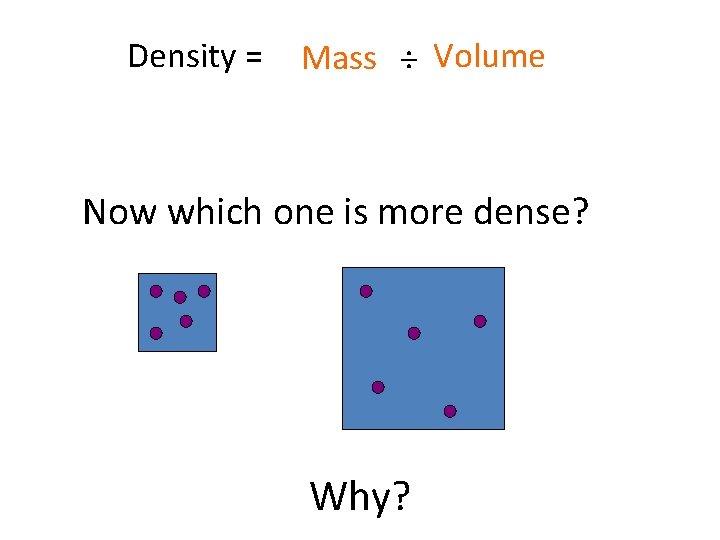
Density = Mass ÷ Volume Now which one is more dense? Why?

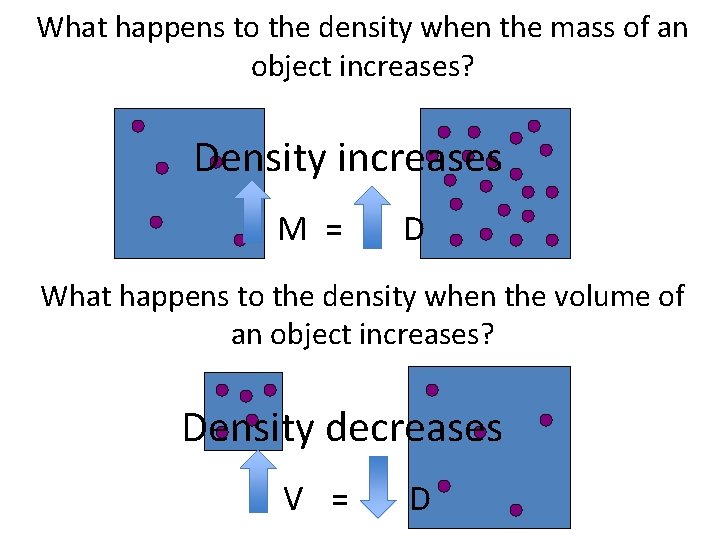
What happens to the density when the mass of an object increases? Density increases M = D What happens to the density when the volume of an object increases? Density decreases V = D

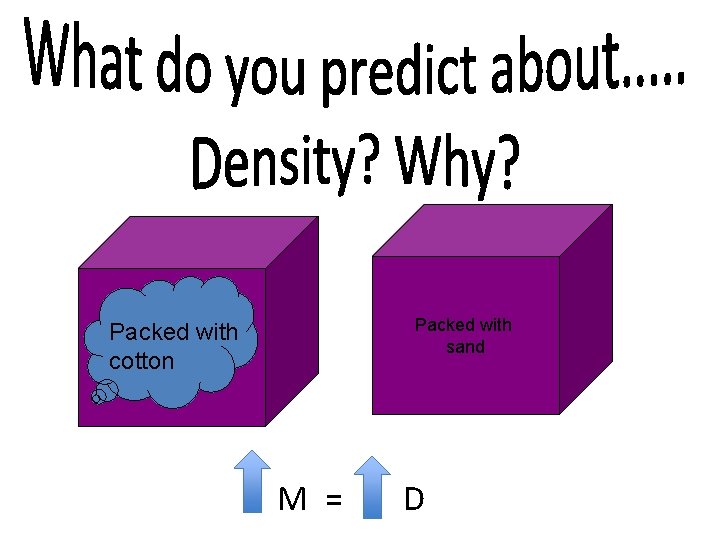
Packed with sand Packed with cotton M = D

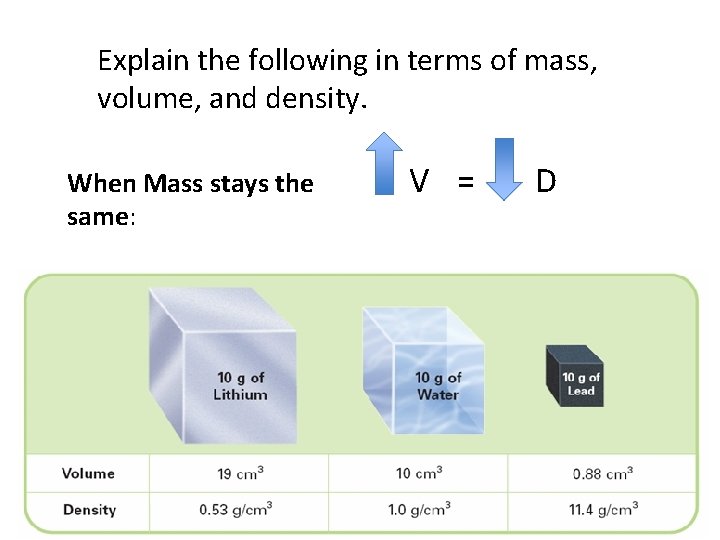
Explain the following in terms of mass, volume, and density. When Mass stays the same: V = D

Gummy Bear Density Lab What happens to the density of a gummy when left in water overnight? Why?

Density and Floating

An object will float if it is _____ than a surrounding liquid. More or Less dense? ? LESS DENSE!!!!

Will an object with a density of 0. 7 g/cm³ float or sink in water? • An object that has a density of 0. 7 g/cm³ will float in water (1 g/cm³) because it is less dense than water