Chapter 9 Molecular Geometry Molecular Shapes n Lewis















- Slides: 65

Chapter 9 Molecular Geometry

Molecular Shapes n Lewis Structures do not account for shape of molecules rather they only show the number and types of bonds between atoms

Molecular Shapes n The overall shape is determined by the bond angle, the angle made by the lines joining the nuclei of the atoms in a molecule

Molecular Shapes n We will focus on atoms with ABn format, a central atom A has n B atoms bonded to it

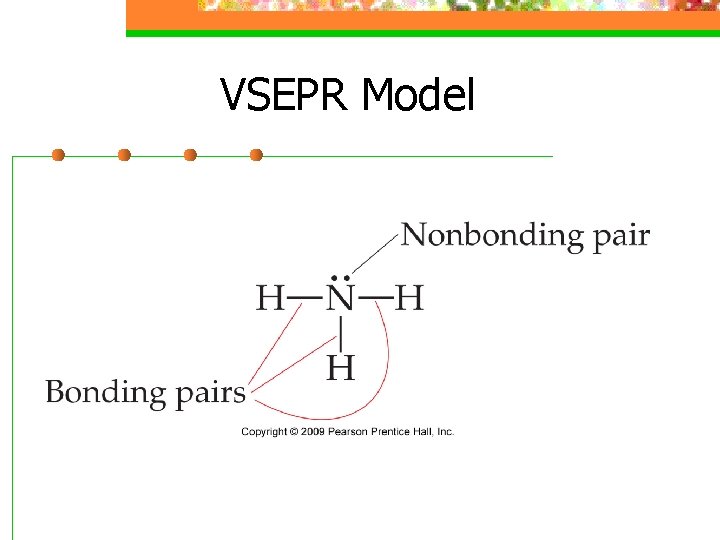
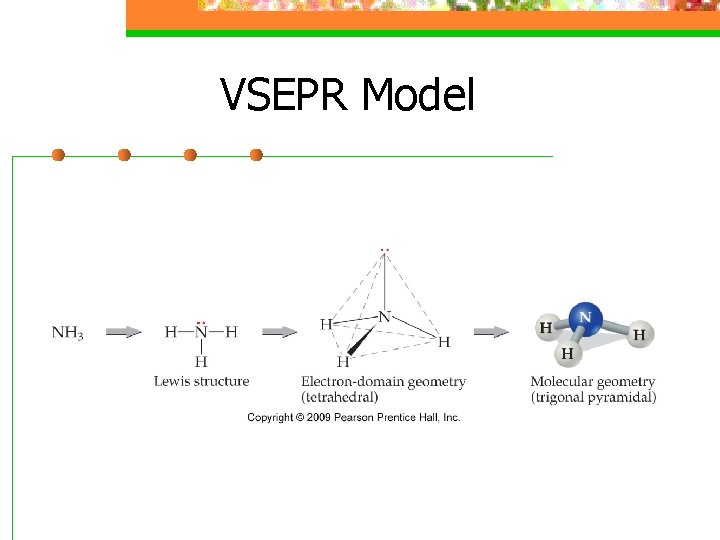
VSEPR Model n An electron domain consists of nonbonding pairs, single bonds, or multiple bonds

VSEPR Model

VSEPR Model n Electron domains are negatively charged, so they repel each other, and stay out of each other’s way

VSEPR Model n the best arrangement of a given number of electron domains is the one that minimizes the repulsions among them

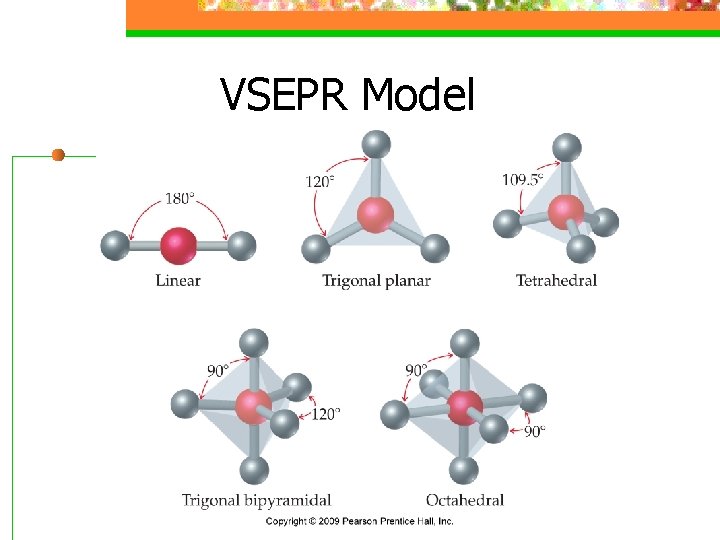
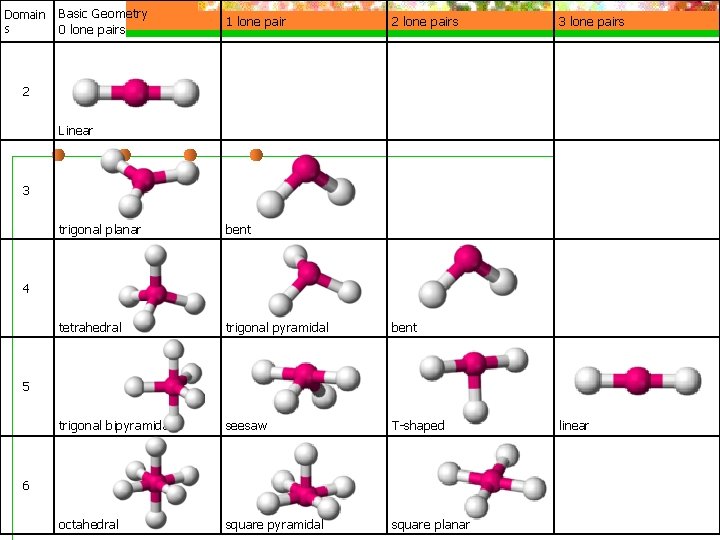
VSEPR Model n The shape of the substance in the ABn format depends on the number of electron domains surrounding the central atom (Electrondomain geometry)

VSEPR Model n Molecular shape describes the arrangement of atoms, not the electron domains (molecular geometry)

VSEPR Model n To Predict Shapes: n 1. Draw Lewis structure and count e-d n 2. Determine e-d geometry n 3. Predict molecular geometry

VSEPR Model n See table

VSEPR Model

Domain s Basic Geometry 0 lone pairs 1 lone pair 2 lone pairs 3 lone pairs Linear trigonal planar bent tetrahedral trigonal pyramidal bent trigonal bipyramidal seesaw T-shaped linear octahedral square pyramidal square planar 2 3 4 5 6

VSEPR Model

Sample Ex. 8. 1 -8. 2 n n n n Use VSEPR to predict shape for the following substances: O 3 Sn. Cl 3 Se. Cl 2 CO 32 IF 5 Cl. F 3 ICl 4 -

VSEPR Model n video

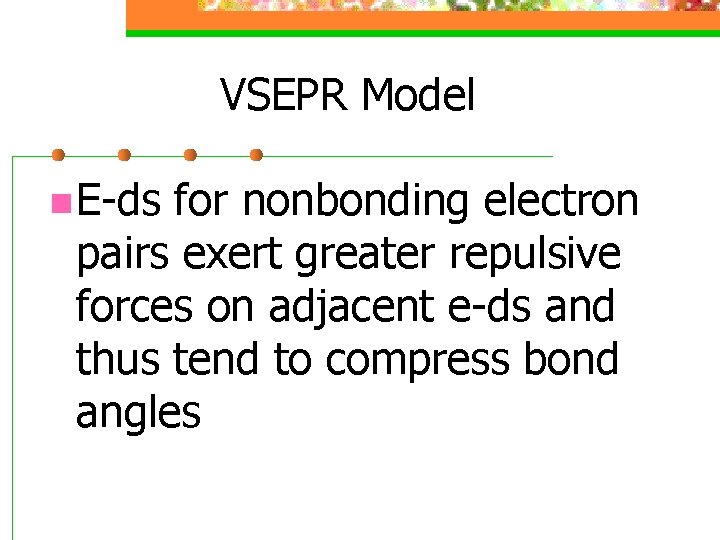
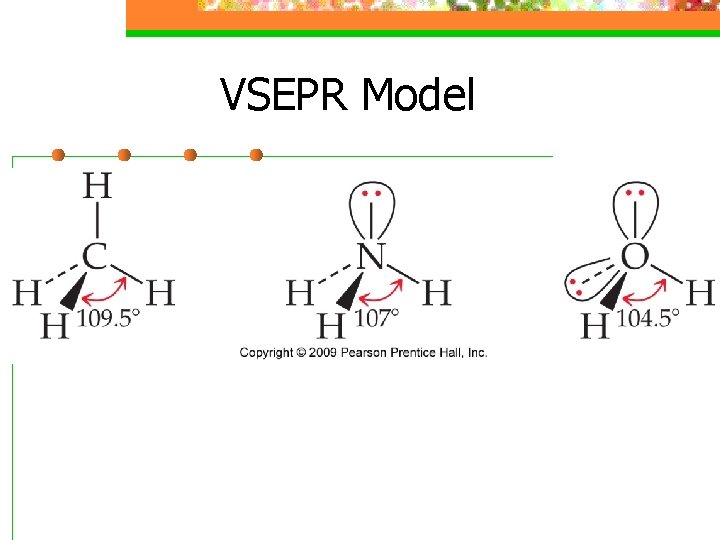
VSEPR Model n Nonbonding Electrons and Multiple Bonds can affect bond angles

VSEPR Model n E-ds for nonbonding electron pairs exert greater repulsive forces on adjacent e-ds and thus tend to compress bond angles

VSEPR Model

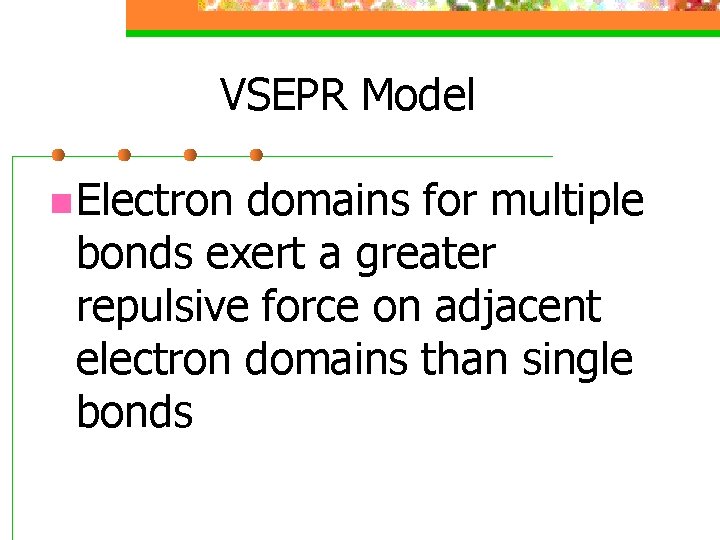
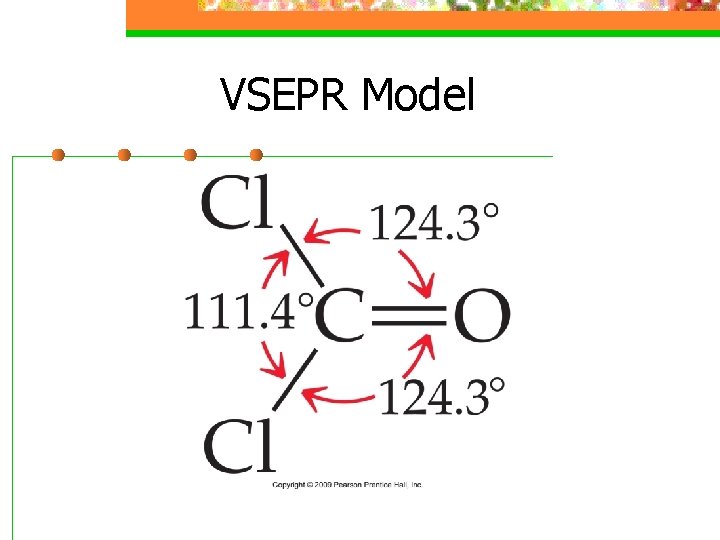
VSEPR Model n Electron domains for multiple bonds exert a greater repulsive force on adjacent electron domains than single bonds

VSEPR Model

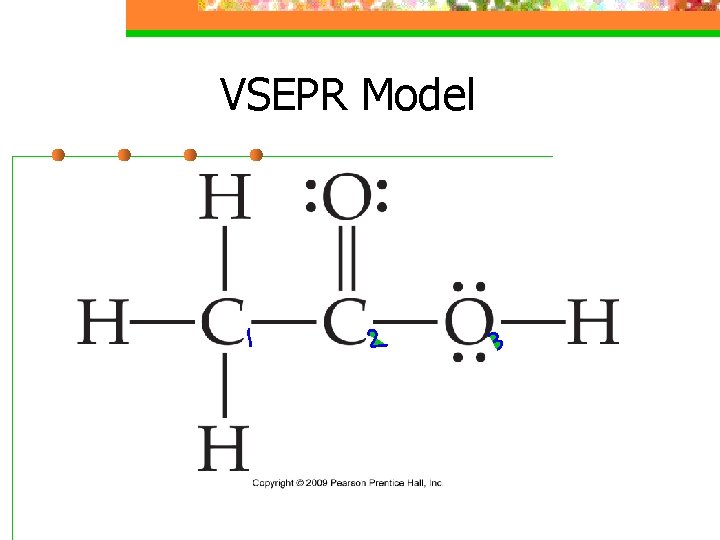
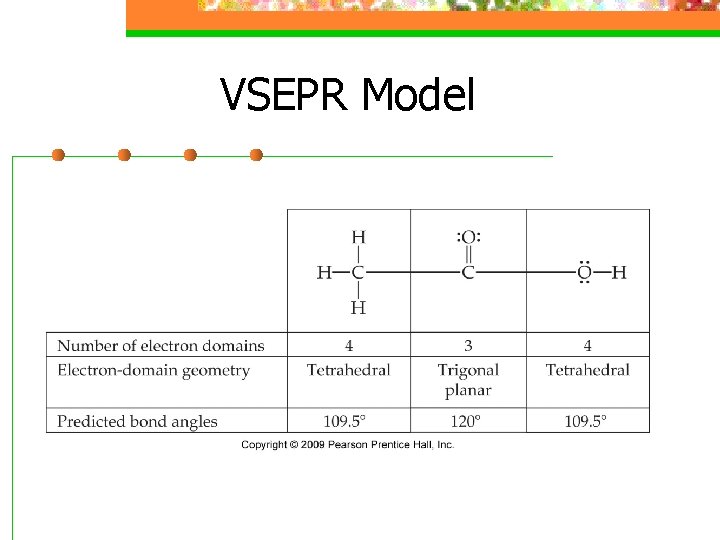
VSEPR Model n VSEPR can be applied to larger molecules as well n The shape can be predicted around individual atoms

VSEPR Model

VSEPR Model

VSEPR Model

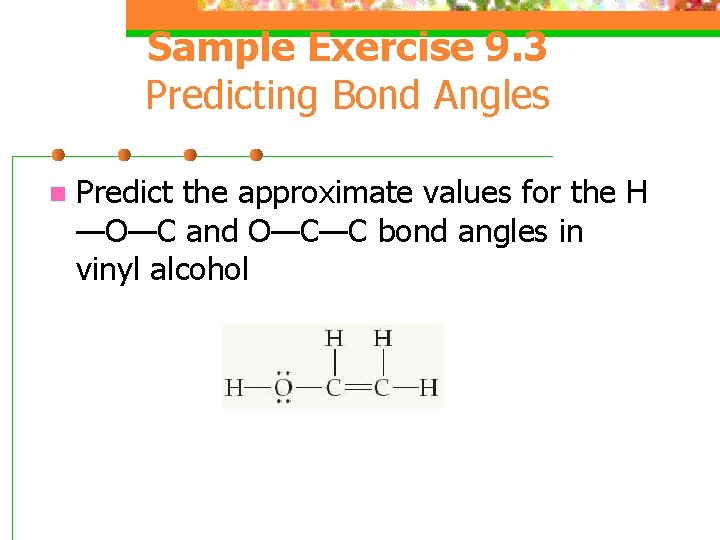
Sample Exercise 9. 3 Predicting Bond Angles n Predict the approximate values for the H —O—C and O—C—C bond angles in vinyl alcohol

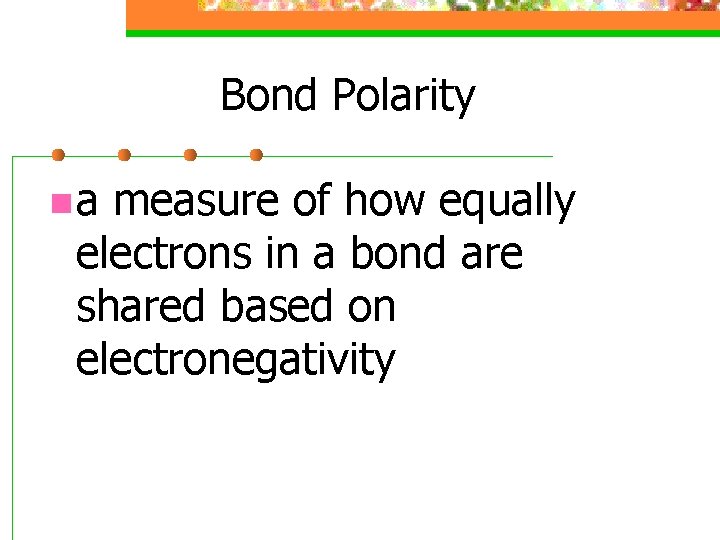
Bond Polarity n a measure of how equally electrons in a bond are shared based on electronegativity

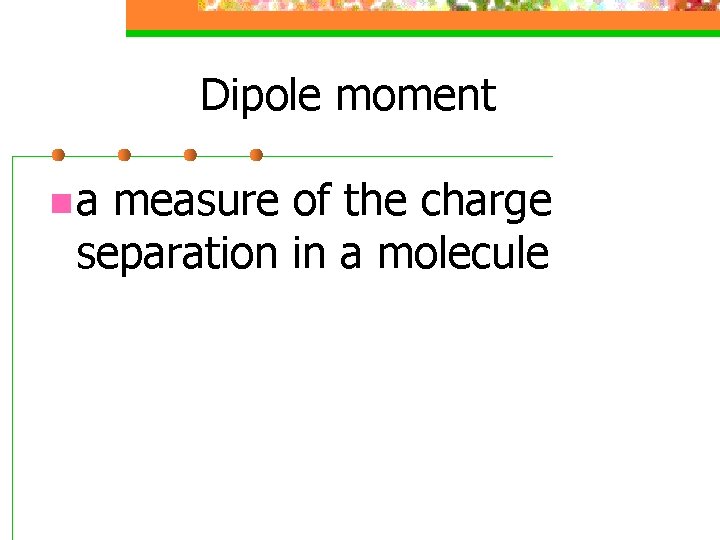
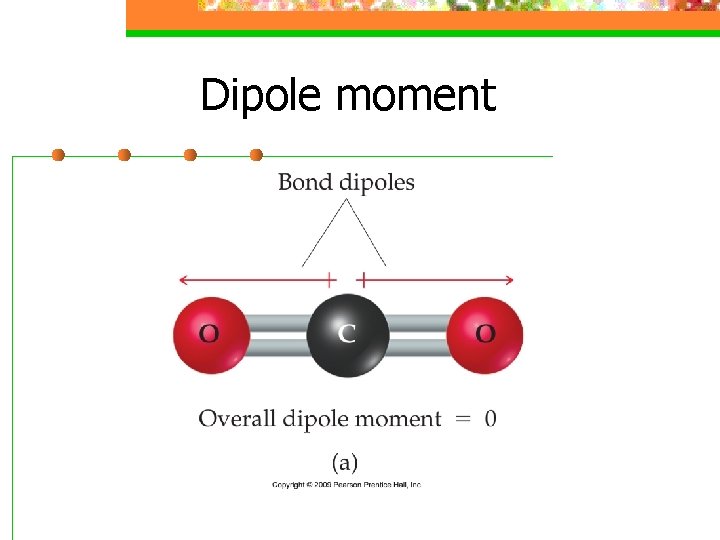
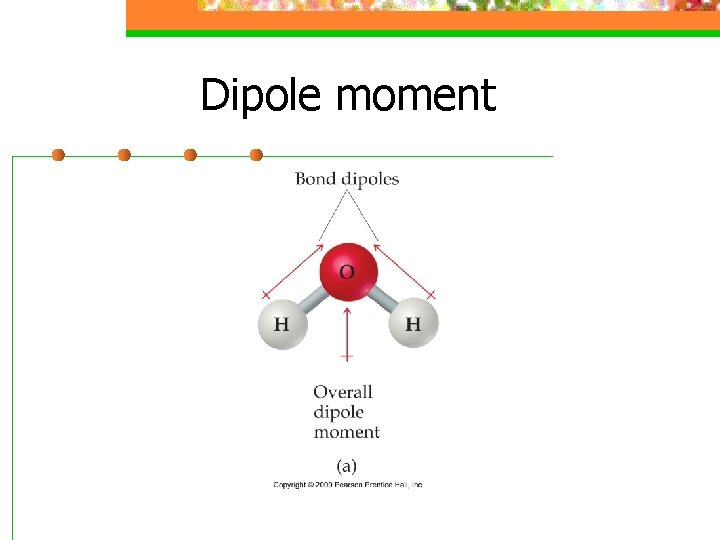
Dipole moment n a measure of the charge separation in a molecule

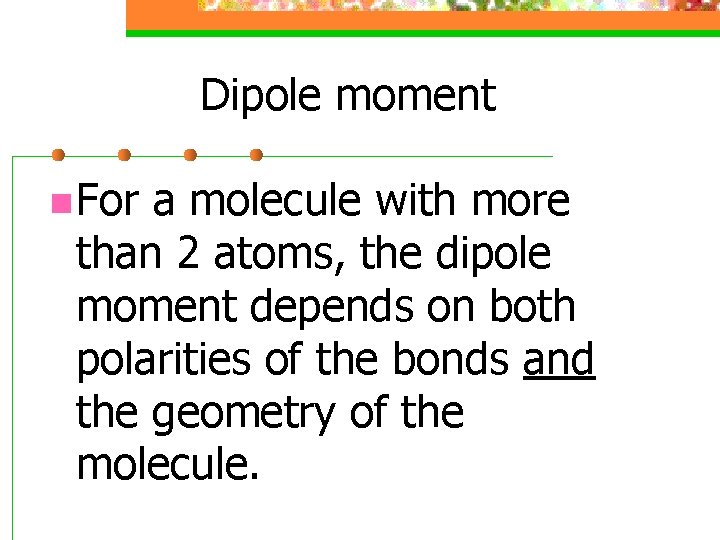
Dipole moment n For a molecule with more than 2 atoms, the dipole moment depends on both polarities of the bonds and the geometry of the molecule.

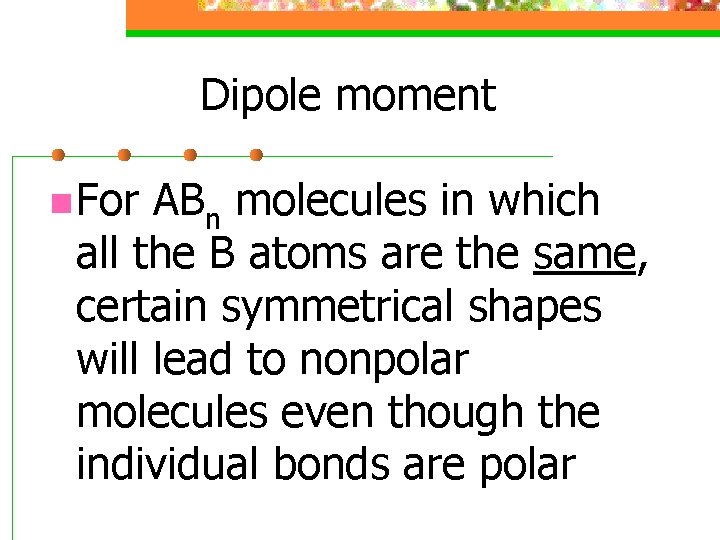
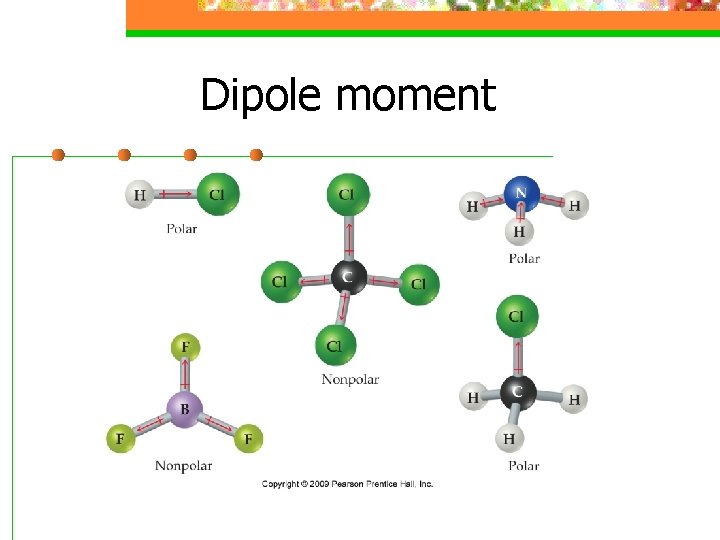
Dipole moment n For ABn molecules in which all the B atoms are the same, certain symmetrical shapes will lead to nonpolar molecules even though the individual bonds are polar

Dipole moment n Nonpolar symmetrical shapes are linear, trigonal planar, tetrahedral, square planar, trigonal bipyramidal, and octahedral

Dipole moment

Dipole moment

Dipole moment

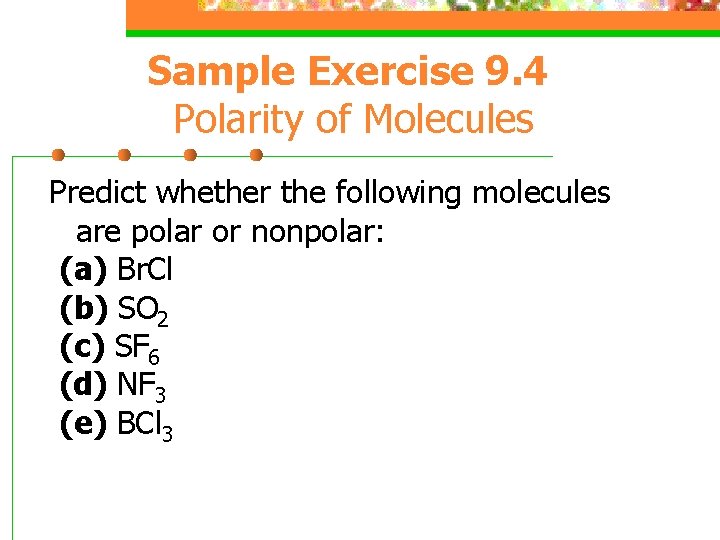
Sample Exercise 9. 4 Polarity of Molecules Predict whether the following molecules are polar or nonpolar: (a) Br. Cl (b) SO 2 (c) SF 6 (d) NF 3 (e) BCl 3

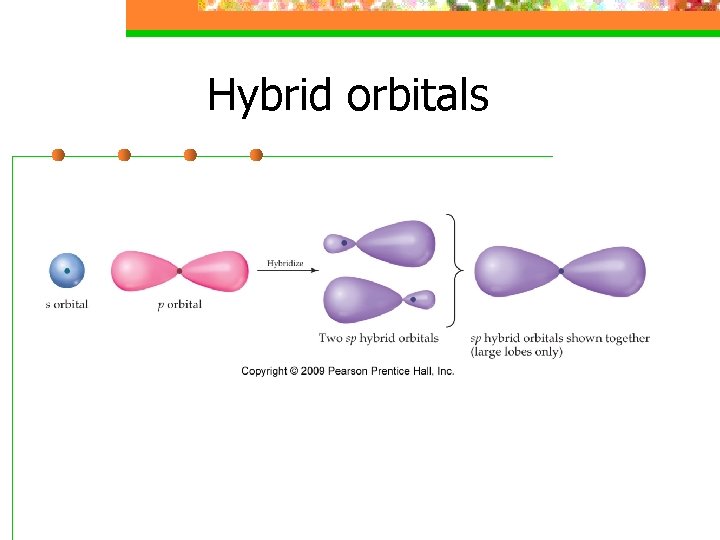
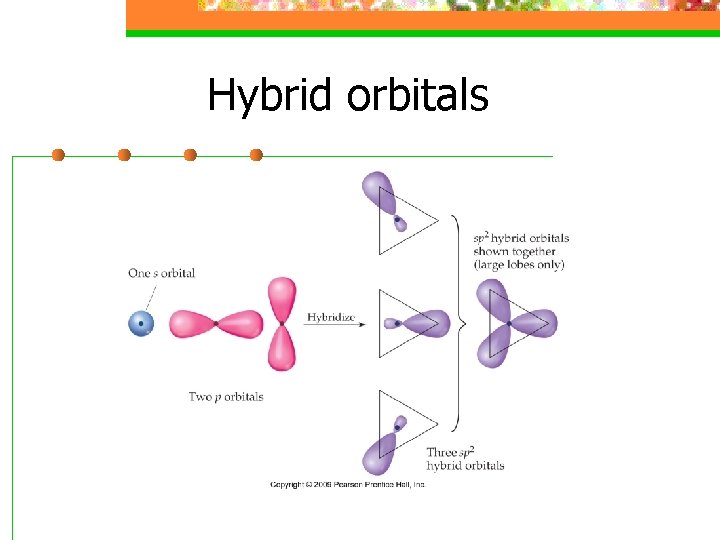
Hybrid orbitals n Hybridization is a process that occurs when atomic orbitals mix to form new orbitals called hybrid orbitals and form bonds

Hybrid orbitals

Hybrid orbitals

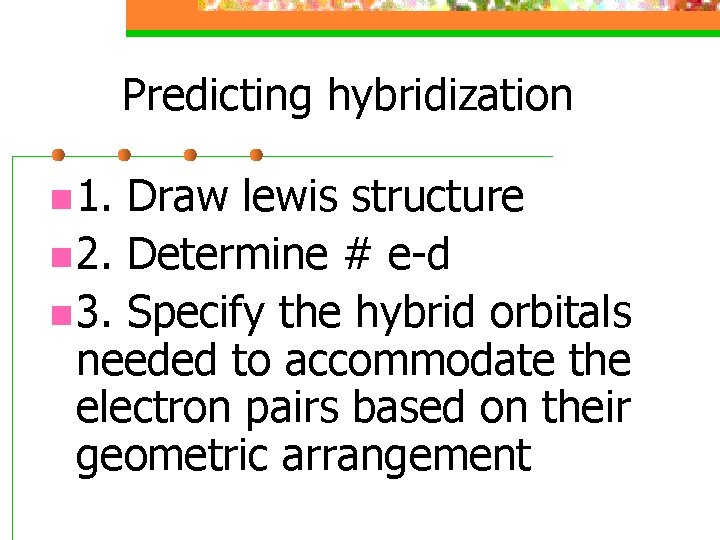
Predicting hybridization n 1. Draw lewis structure n 2. Determine # e-d n 3. Specify the hybrid orbitals needed to accommodate the electron pairs based on their geometric arrangement

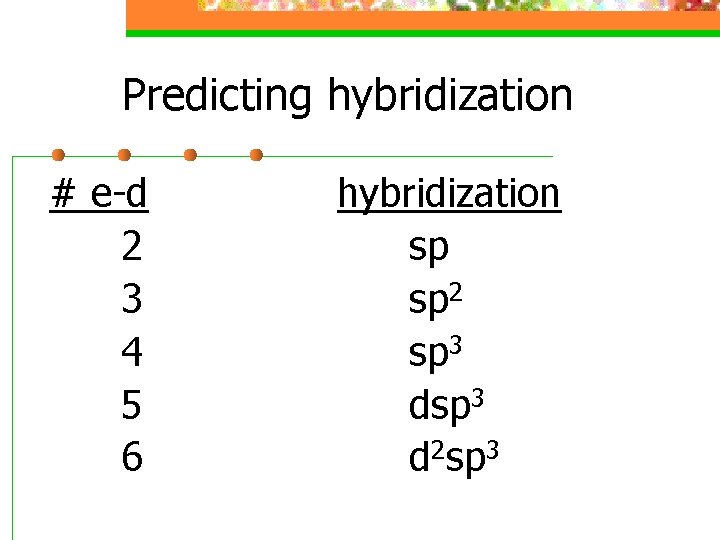
Predicting hybridization # e-d 2 3 4 5 6 hybridization sp sp 2 sp 3 d 2 sp 3

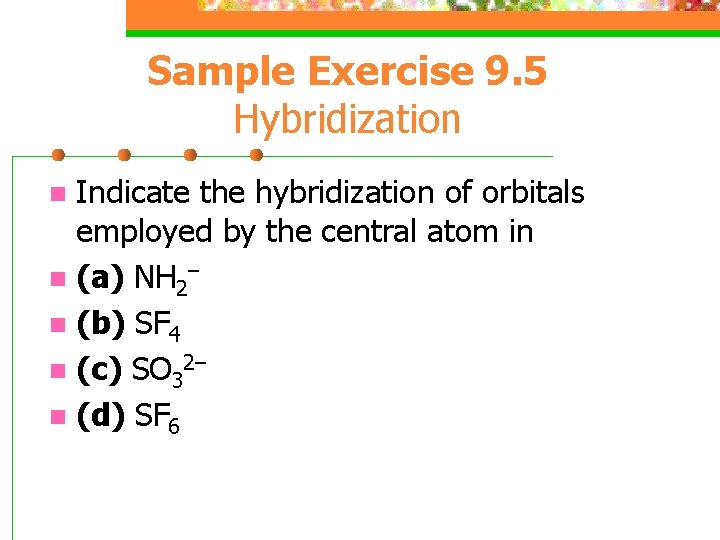
Sample Exercise 9. 5 Hybridization Indicate the hybridization of orbitals employed by the central atom in n (a) NH 2– n (b) SF 4 n (c) SO 32– n (d) SF 6 n

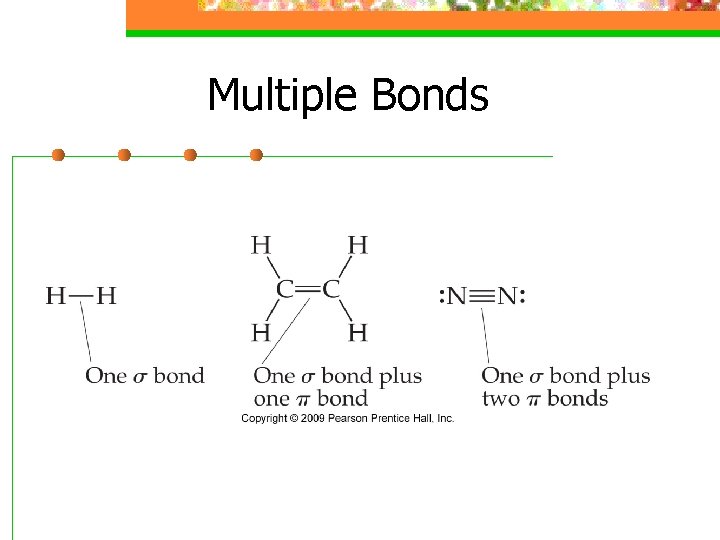
Multiple Bonds n All of the single bonds we have looked at are called sigma (s) bonds, where there is an overlap in the middle of the region of s and p orbital

Multiple Bonds n To describe multiple bonds, we must look at the side to side overlapping of 2 p orbitals, called a pi (p) bond

Multiple Bonds n A double bond is one s and one p bond

Multiple Bonds n A triple bond is one s and two p bonds bond

Multiple Bonds

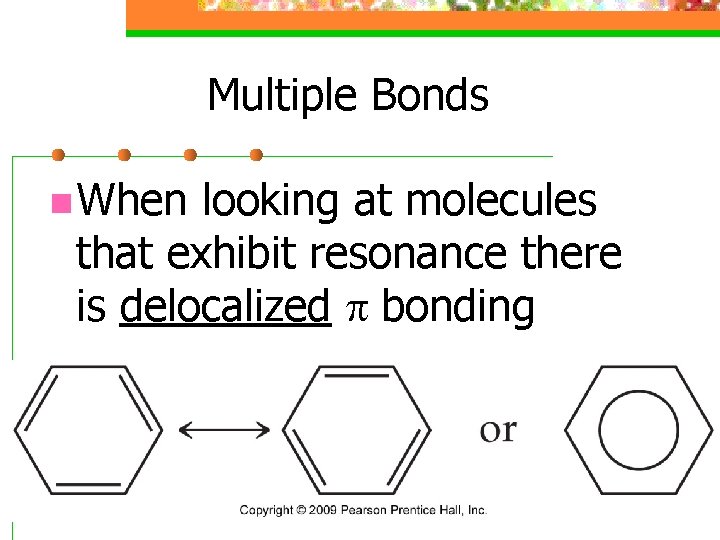
Multiple Bonds n When looking at molecules that exhibit resonance there is delocalized p bonding

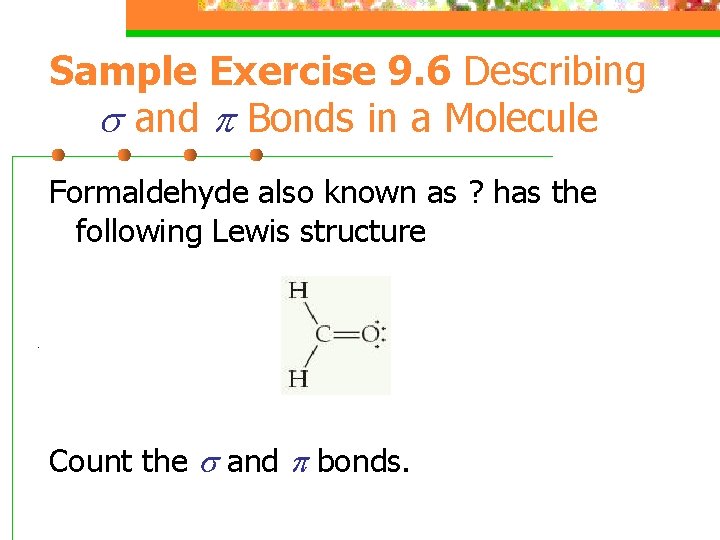
Sample Exercise 9. 6 Describing and Bonds in a Molecule Formaldehyde also known as ? has the following Lewis structure . Count the and bonds.

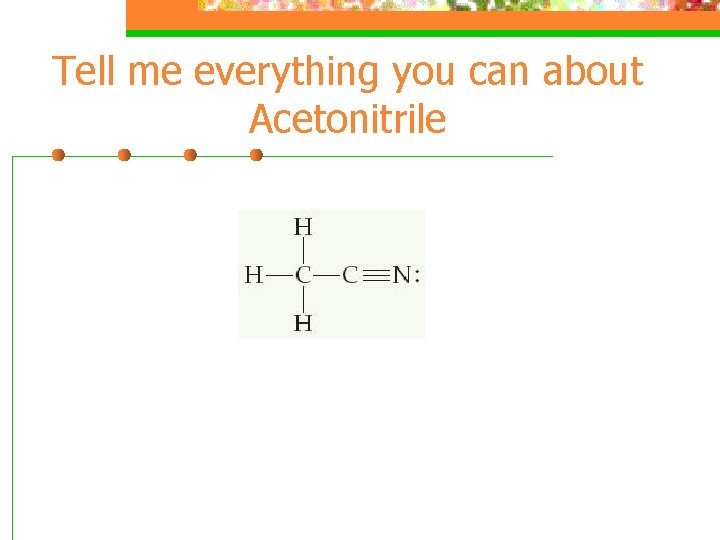
Tell me everything you can about Acetonitrile

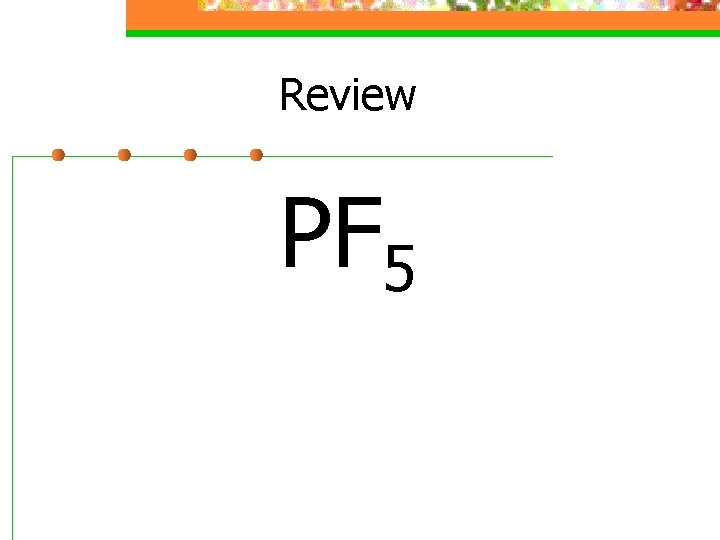
Review PF 5

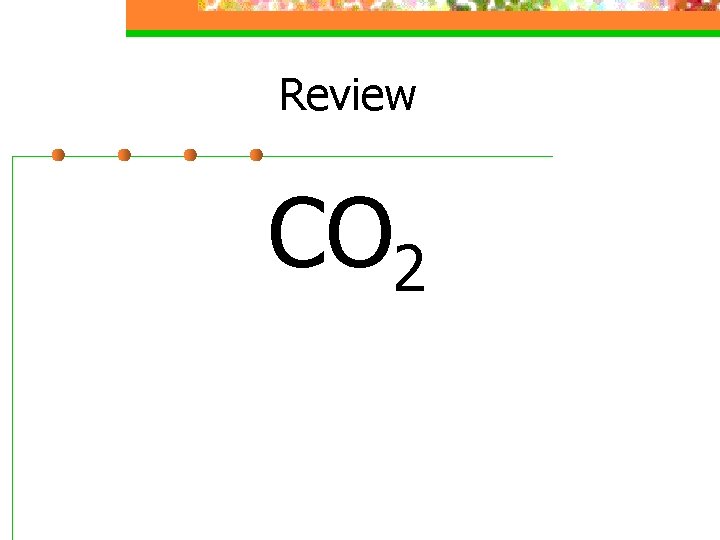
Review CO 2

Review ICl 4 -

Review As. H 3

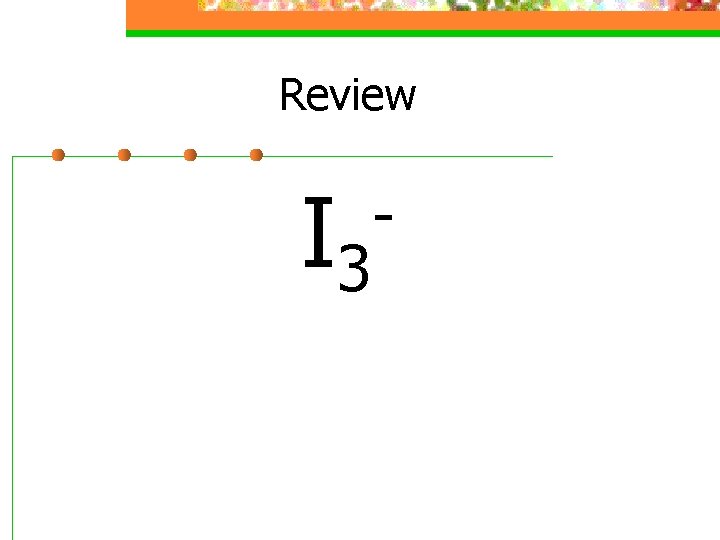
Review I 3 -

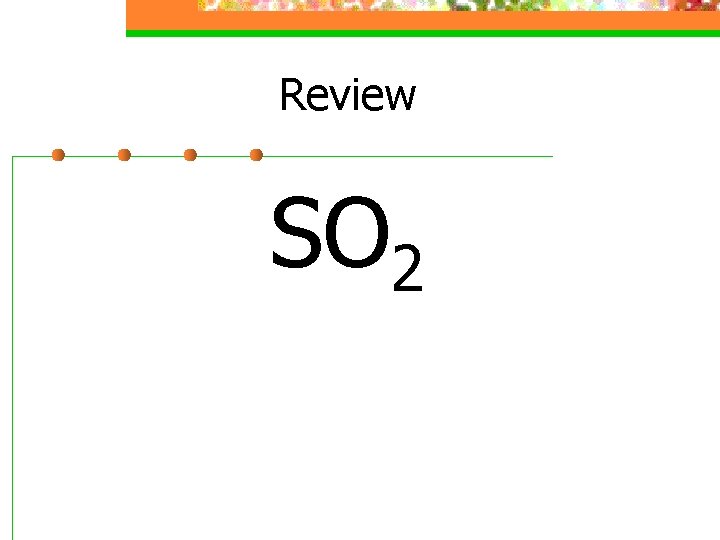
Review SO 2

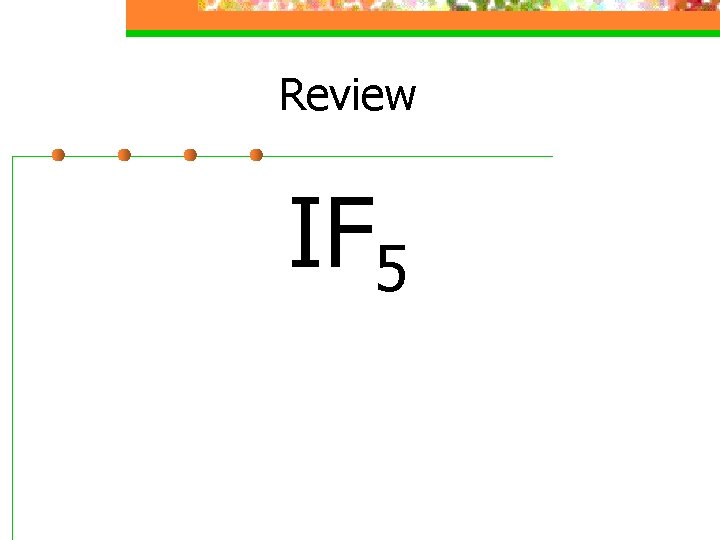
Review IF 5

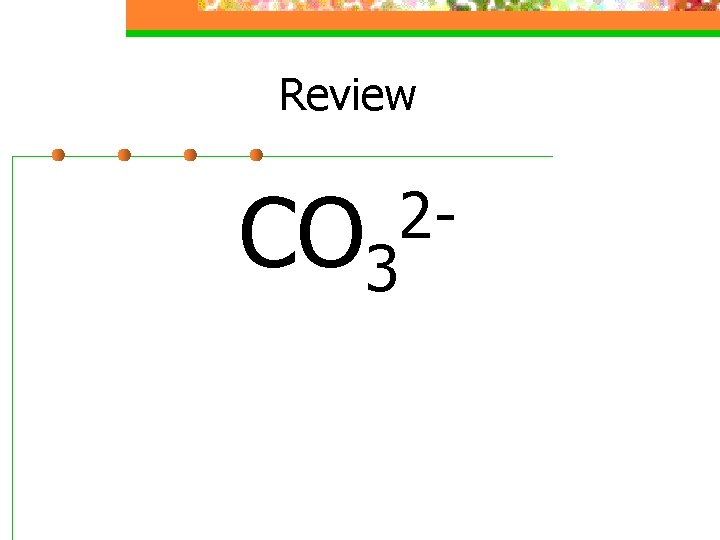
Review CO 3 2 -

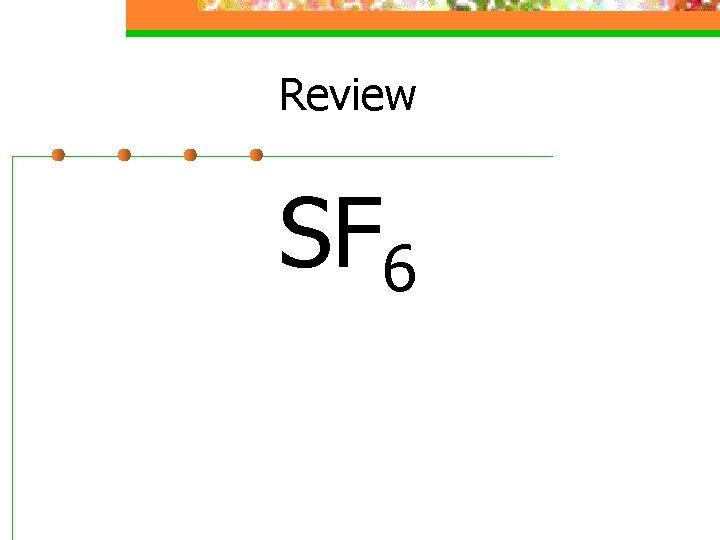
Review SF 6

Review Cl. O 4 -

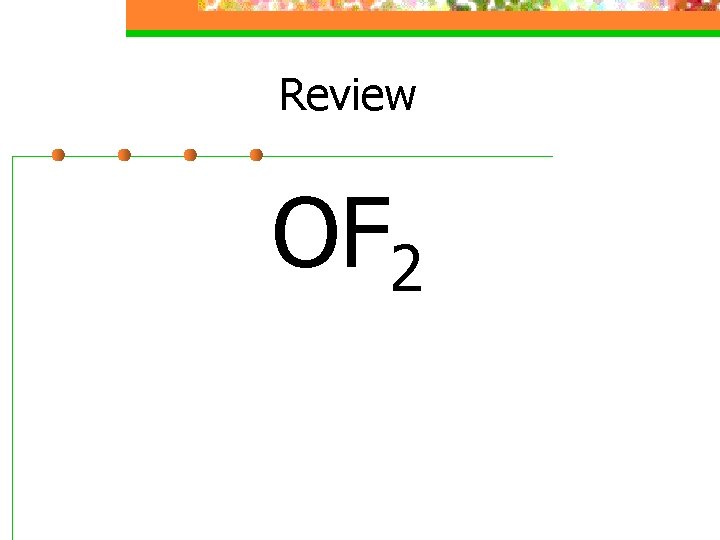
Review OF 2

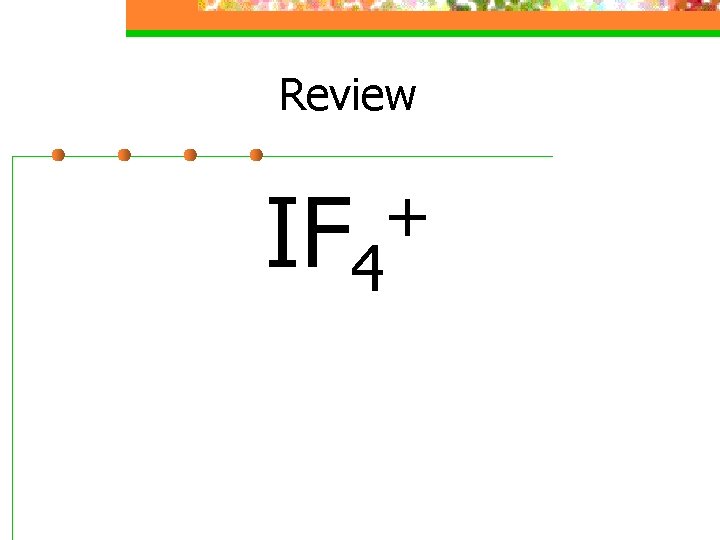
Review IF 4 +

Review Cl. F 3

Review NCS

Review Directions: Predict the molecular geometry, bond angle, hybridization, s and p bonds, and polar/nonpolar for the following molecule : Methanoic acid Methyl Amine Ethyne