Molecular Geometry Valence Shell Electron Pair Repulsion VSEPR
- Slides: 12

Molecular Geometry

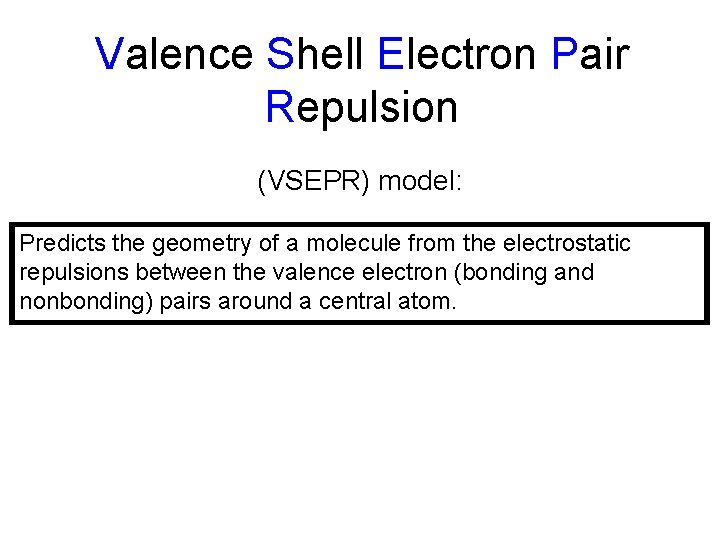
Valence Shell Electron Pair Repulsion (VSEPR) model: Predicts the geometry of a molecule from the electrostatic repulsions between the valence electron (bonding and nonbonding) pairs around a central atom.

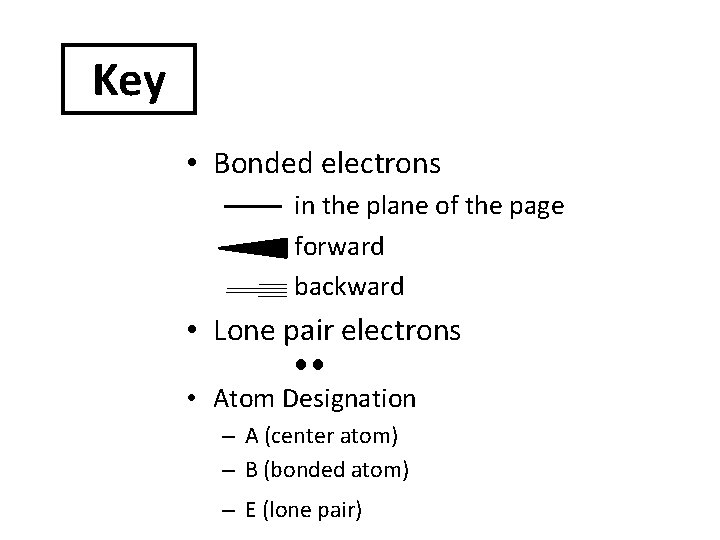
Key • Bonded electrons in the plane of the page forward backward • Lone pair electrons • Atom Designation – A (center atom) – B (bonded atom) – E (lone pair)

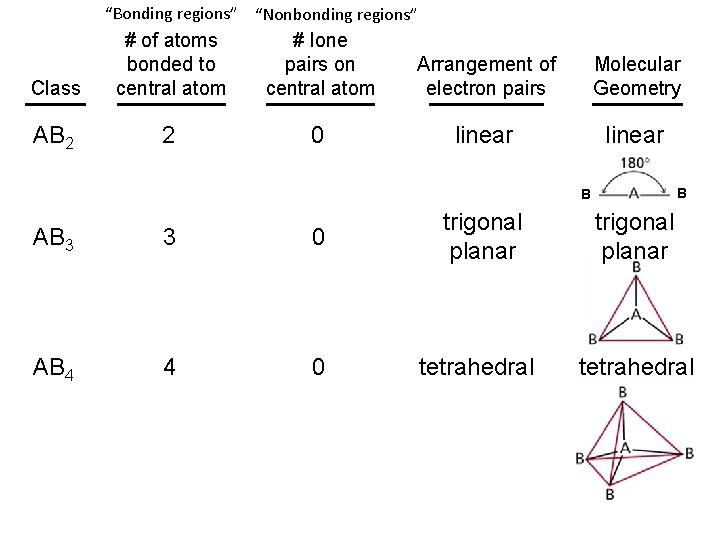
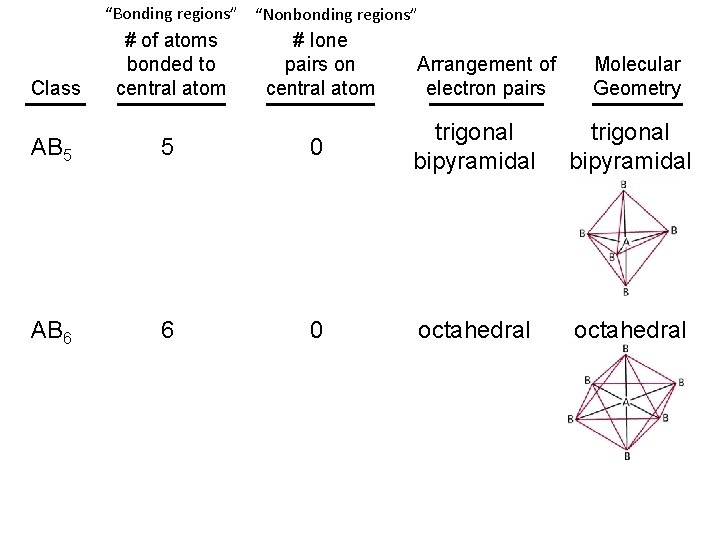
“Bonding regions” “Nonbonding regions” Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB 2 2 0 linear B B AB 3 3 0 trigonal planar AB 4 4 0 tetrahedral trigonal planar tetrahedral

“Bonding regions” “Nonbonding regions” Class # of atoms bonded to central atom # lone pairs on central atom AB 5 5 0 trigonal bipyramidal AB 6 6 0 octahedral Arrangement of electron pairs Molecular Geometry

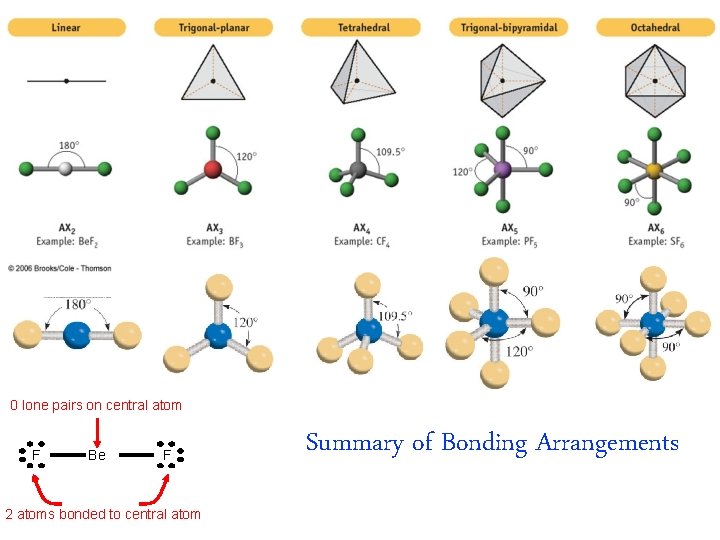
0 lone pairs on central atom F Be F 2 atoms bonded to central atom Summary of Bonding Arrangements

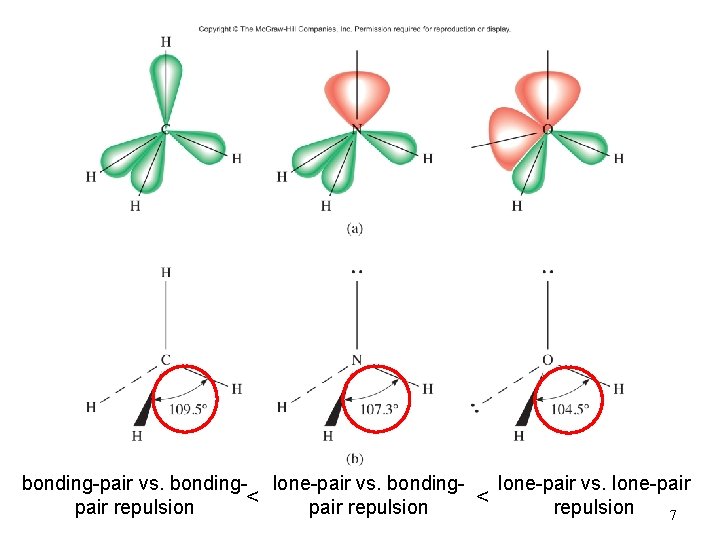
bonding-pair vs. bonding- lone-pair vs. bonding< pair repulsion < lone-pair vs. lone-pair repulsion 7

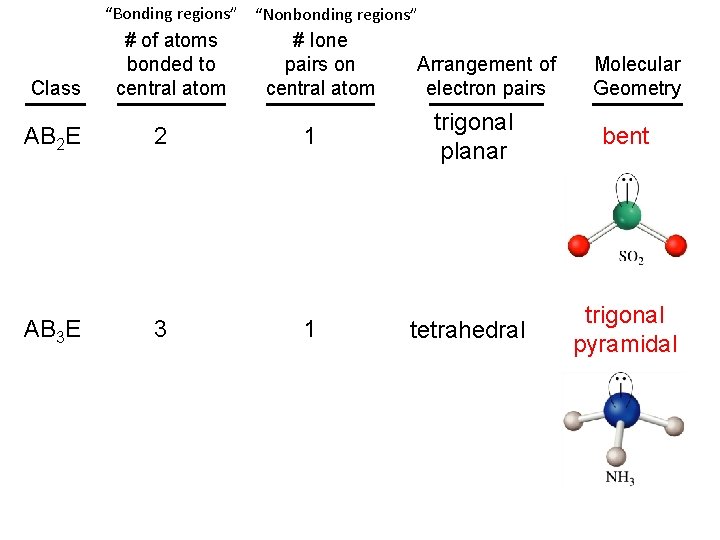
“Bonding regions” “Nonbonding regions” Class AB 2 E AB 3 E # of atoms bonded to central atom 2 3 # lone pairs on central atom 1 1 Arrangement of electron pairs Molecular Geometry trigonal planar bent tetrahedral trigonal pyramidal

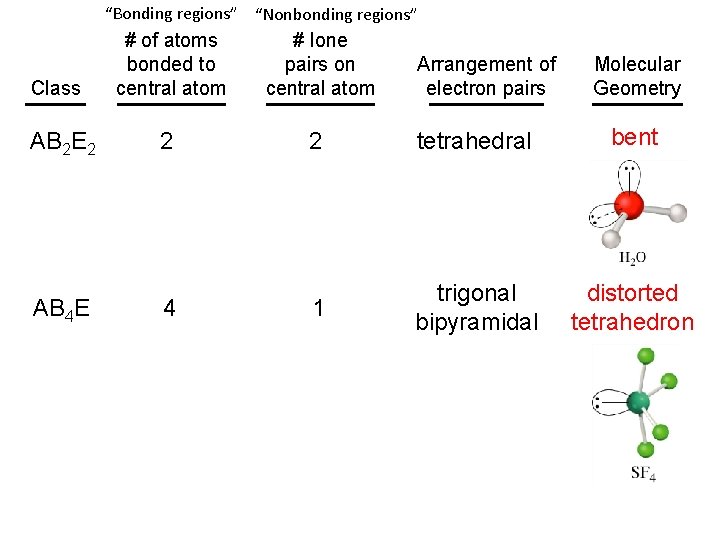
“Bonding regions” “Nonbonding regions” # of atoms bonded to central atom # lone pairs on central atom AB 2 E 2 2 2 tetrahedral bent AB 4 E 4 1 trigonal bipyramidal distorted tetrahedron Class Arrangement of electron pairs Molecular Geometry

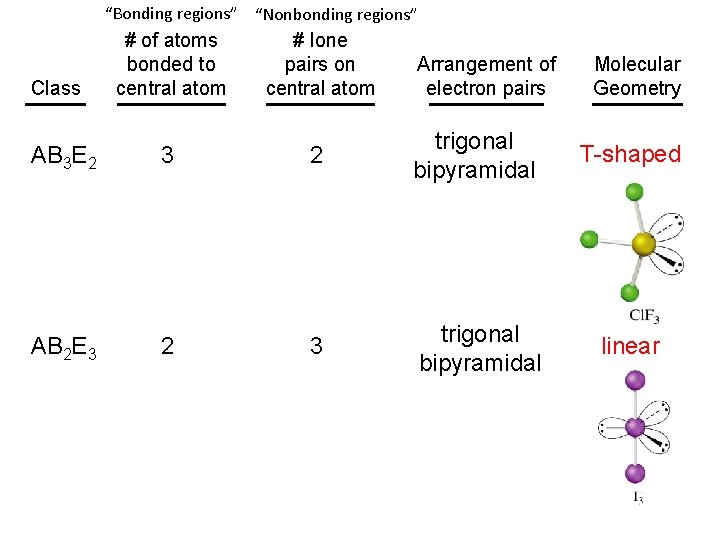
“Bonding regions” “Nonbonding regions” # of atoms bonded to central atom # lone pairs on central atom AB 3 E 2 3 2 trigonal bipyramidal T-shaped AB 2 E 3 2 3 trigonal bipyramidal linear Class Arrangement of electron pairs Molecular Geometry

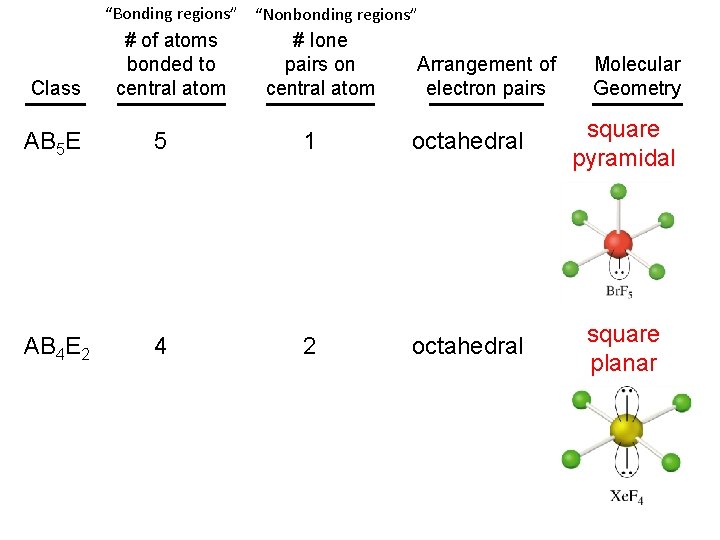
“Bonding regions” “Nonbonding regions” Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AB 5 E 5 1 octahedral square pyramidal AB 4 E 2 4 2 octahedral square planar

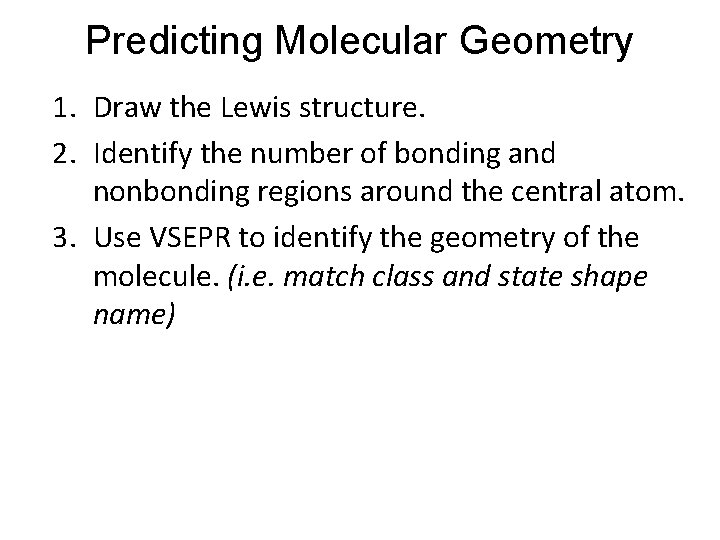
Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Identify the number of bonding and nonbonding regions around the central atom. 3. Use VSEPR to identify the geometry of the molecule. (i. e. match class and state shape name)