Molecular Geometry VSEPR Theory and Polar Molecules ValenceShell


- Slides: 48

Molecular Geometry VSEPR Theory and Polar Molecules

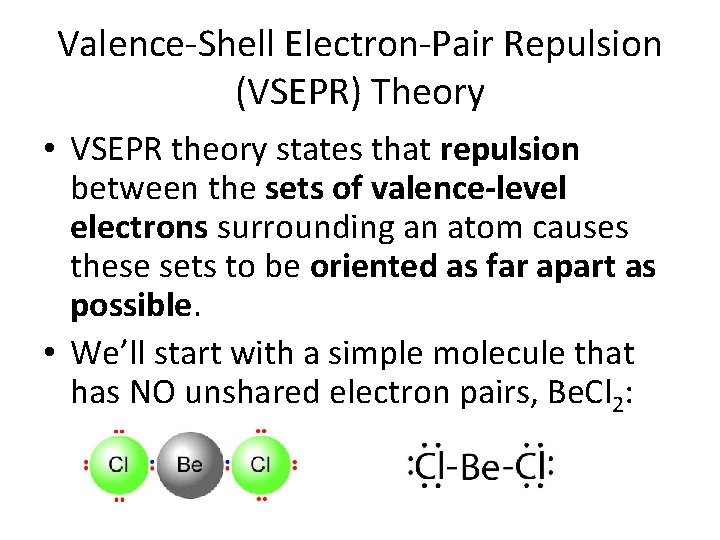
Valence-Shell Electron-Pair Repulsion (VSEPR) Theory • VSEPR theory states that repulsion between the sets of valence-level electrons surrounding an atom causes these sets to be oriented as far apart as possible. • We’ll start with a simple molecule that has NO unshared electron pairs, Be. Cl 2:

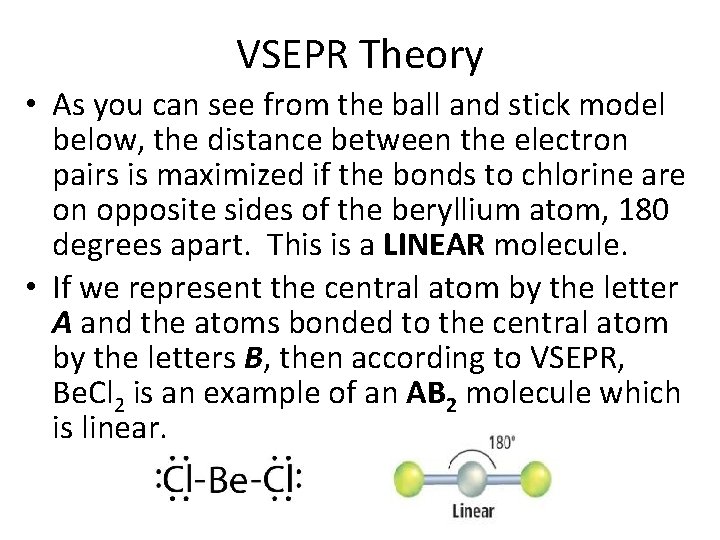
VSEPR Theory • As you can see from the ball and stick model below, the distance between the electron pairs is maximized if the bonds to chlorine are on opposite sides of the beryllium atom, 180 degrees apart. This is a LINEAR molecule. • If we represent the central atom by the letter A and the atoms bonded to the central atom by the letters B, then according to VSEPR, Be. Cl 2 is an example of an AB 2 molecule which is linear.

VSEPR Theory • In the general formula, B can represent a single atom, a group of identical atoms, or a group of different atoms. However, different sizes of B molecules will change the bond angles.

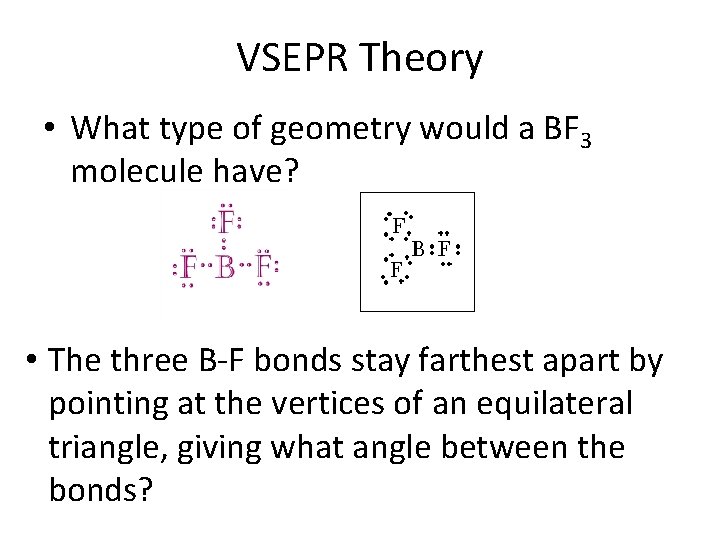
VSEPR Theory • What type of geometry would a BF 3 molecule have? • The three B-F bonds stay farthest apart by pointing at the vertices of an equilateral triangle, giving what angle between the bonds?

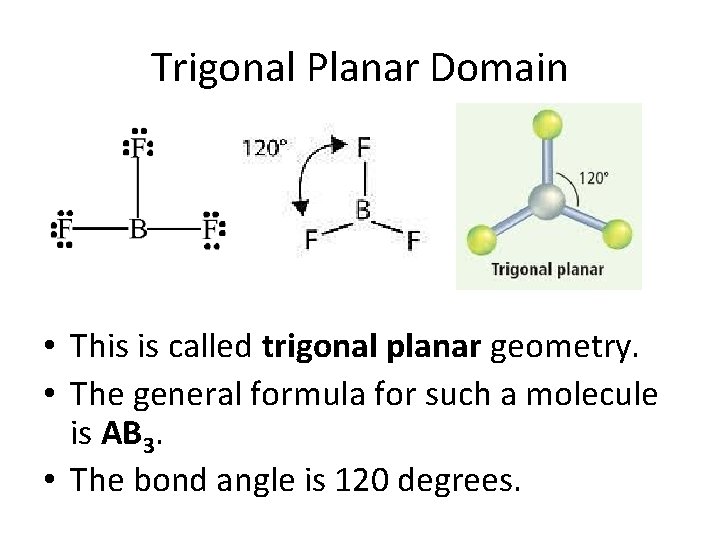
Trigonal Planar Domain • This is called trigonal planar geometry. • The general formula for such a molecule is AB 3. • The bond angle is 120 degrees.

VSEPR Theory • But what about molecular geometries when there are UNBONDED (unshared) pairs of electrons? • VSEPR theory states that these unbonded pairs occupy space around the central atom just like bonded pairs do. • However, observation of real atoms demonstrates that unshared electron pairs repel electrons more strongly than bonded pairs do, so molecular geometries are different when there are unbonded electron pairs.

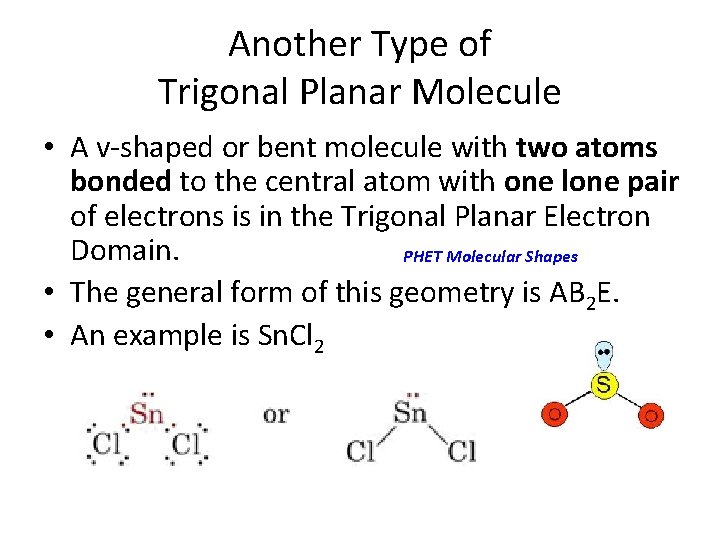
Another Type of Trigonal Planar Molecule • A v-shaped or bent molecule with two atoms bonded to the central atom with one lone pair of electrons is in the Trigonal Planar Electron Domain. PHET Molecular Shapes • The general form of this geometry is AB 2 E. • An example is Sn. Cl 2

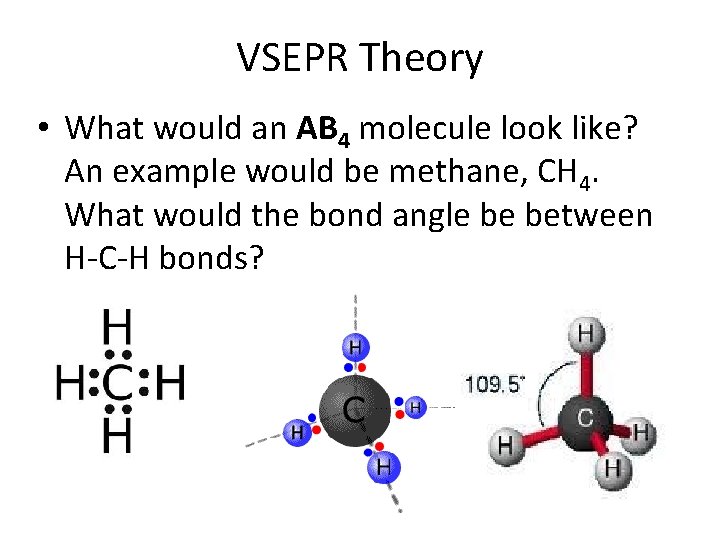
VSEPR Theory • What would an AB 4 molecule look like? An example would be methane, CH 4. What would the bond angle be between H-C-H bonds?

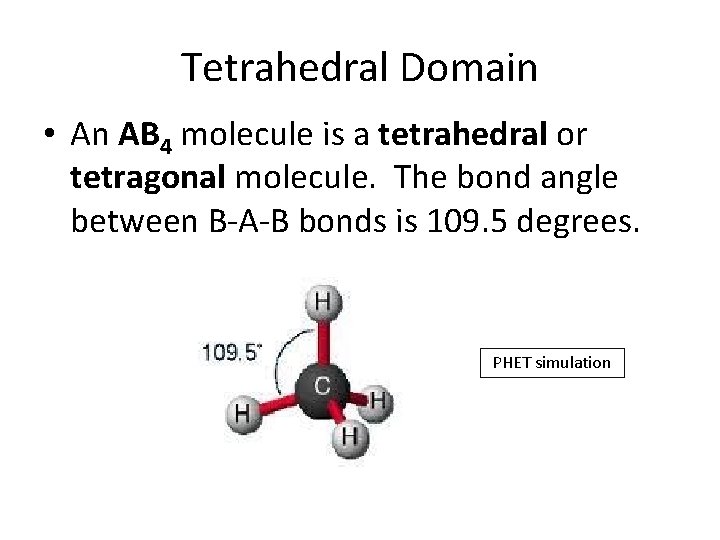
Tetrahedral Domain • An AB 4 molecule is a tetrahedral or tetragonal molecule. The bond angle between B-A-B bonds is 109. 5 degrees. PHET simulation

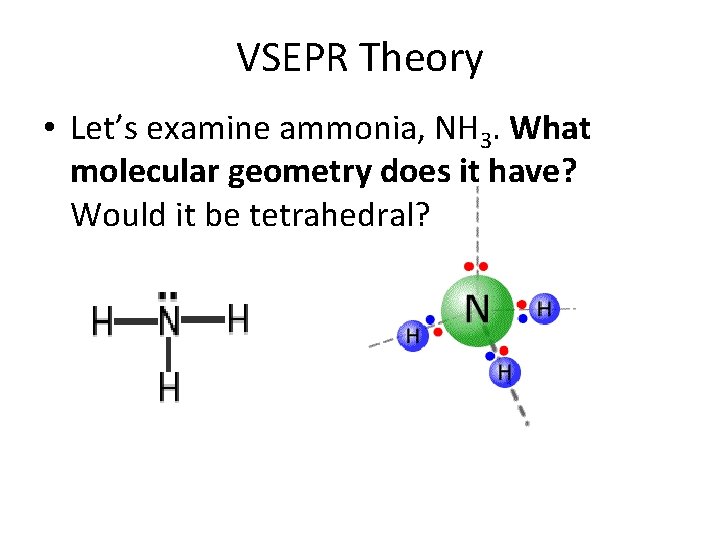
VSEPR Theory • Let’s examine ammonia, NH 3. What molecular geometry does it have? Would it be tetrahedral?

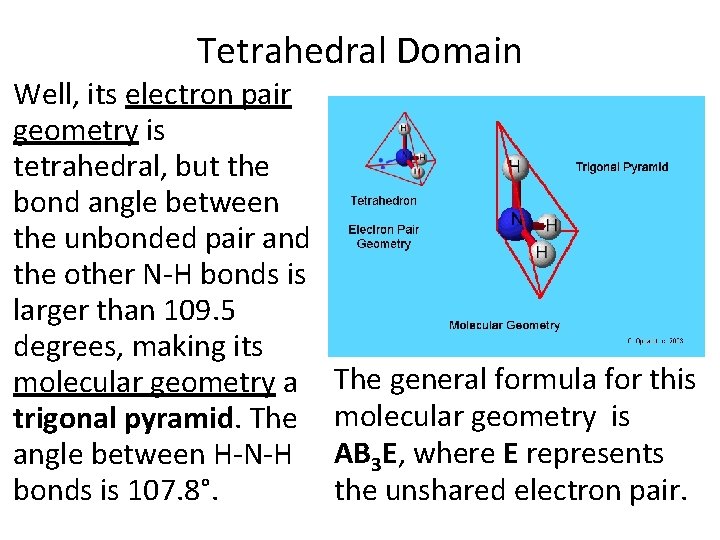
Tetrahedral Domain Well, its electron pair geometry is tetrahedral, but the bond angle between the unbonded pair and the other N-H bonds is larger than 109. 5 degrees, making its molecular geometry a trigonal pyramid. The angle between H-N-H bonds is 107. 8°. The general formula for this molecular geometry is AB 3 E, where E represents the unshared electron pair.

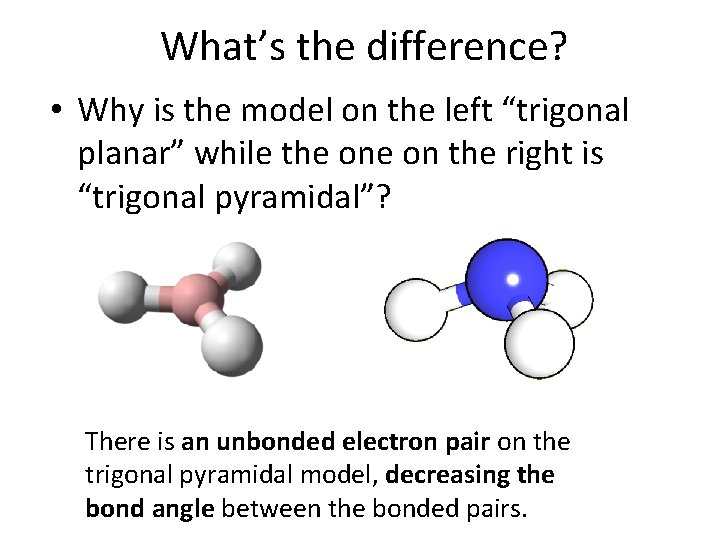
What’s the difference? • Why is the model on the left “trigonal planar” while the on the right is “trigonal pyramidal”? There is an unbonded electron pair on the trigonal pyramidal model, decreasing the bond angle between the bonded pairs.

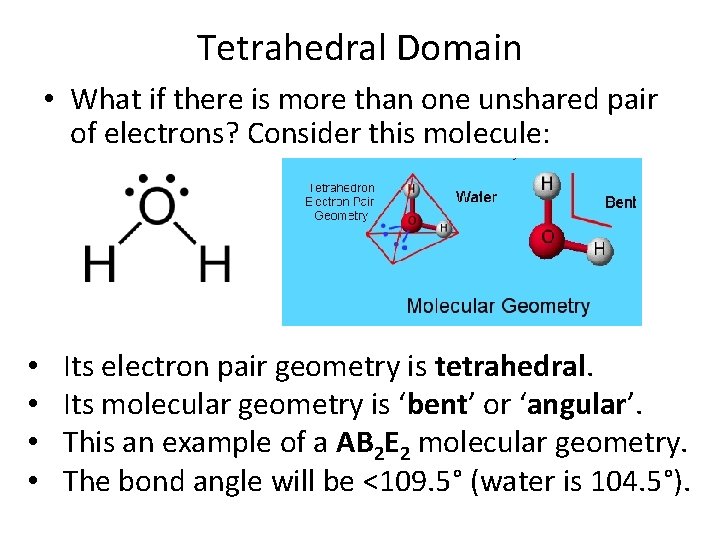
Tetrahedral Domain • What if there is more than one unshared pair of electrons? Consider this molecule: • • Its electron pair geometry is tetrahedral. Its molecular geometry is ‘bent’ or ‘angular’. This an example of a AB 2 E 2 molecular geometry. The bond angle will be <109. 5° (water is 104. 5°).

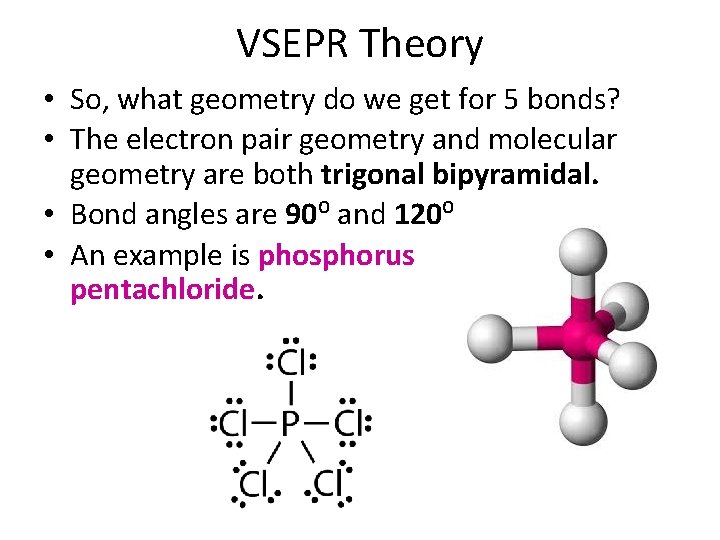
VSEPR Theory • So, what geometry do we get for 5 bonds? • The electron pair geometry and molecular geometry are both trigonal bipyramidal. • Bond angles are 90⁰ and 120⁰. • An example is phosphorus pentachloride.

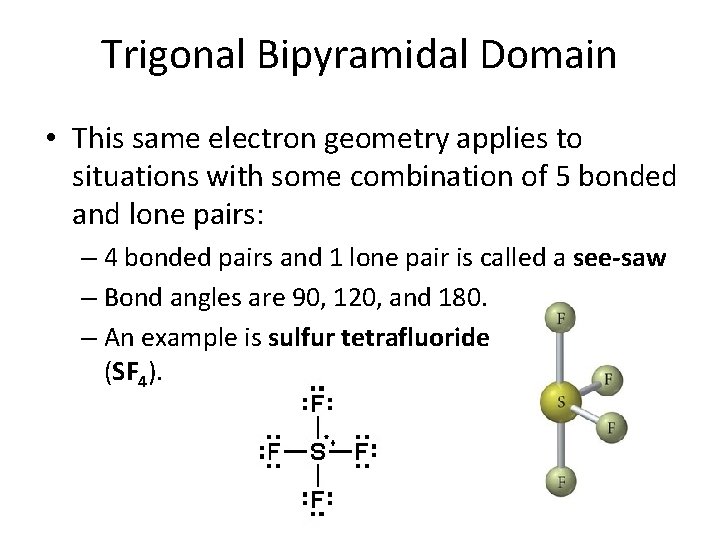
Trigonal Bipyramidal Domain • This same electron geometry applies to situations with some combination of 5 bonded and lone pairs: – 4 bonded pairs and 1 lone pair is called a see-saw – Bond angles are 90, 120, and 180. – An example is sulfur tetrafluoride (SF 4).

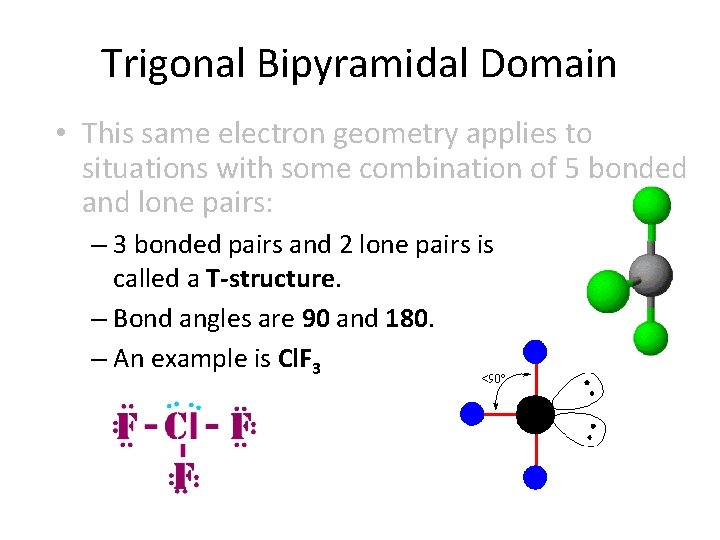
Trigonal Bipyramidal Domain • This same electron geometry applies to situations with some combination of 5 bonded and lone pairs: – 3 bonded pairs and 2 lone pairs is called a T-structure. – Bond angles are 90 and 180. – An example is Cl. F 3

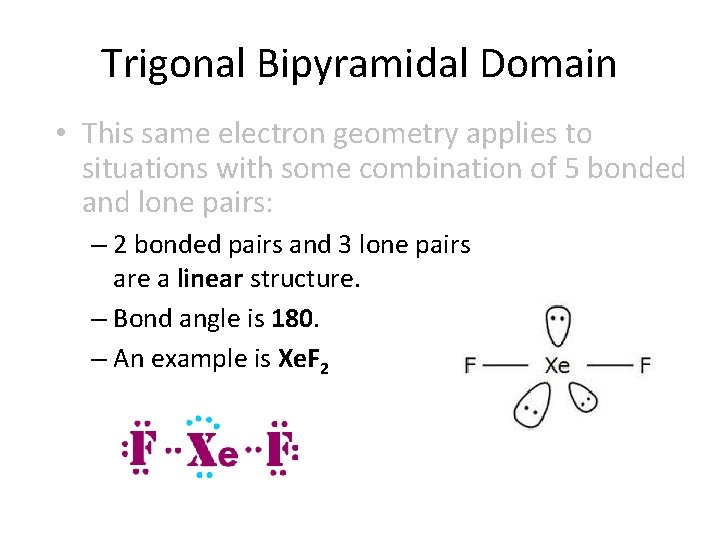
Trigonal Bipyramidal Domain • This same electron geometry applies to situations with some combination of 5 bonded and lone pairs: – 2 bonded pairs and 3 lone pairs are a linear structure. – Bond angle is 180. – An example is Xe. F 2

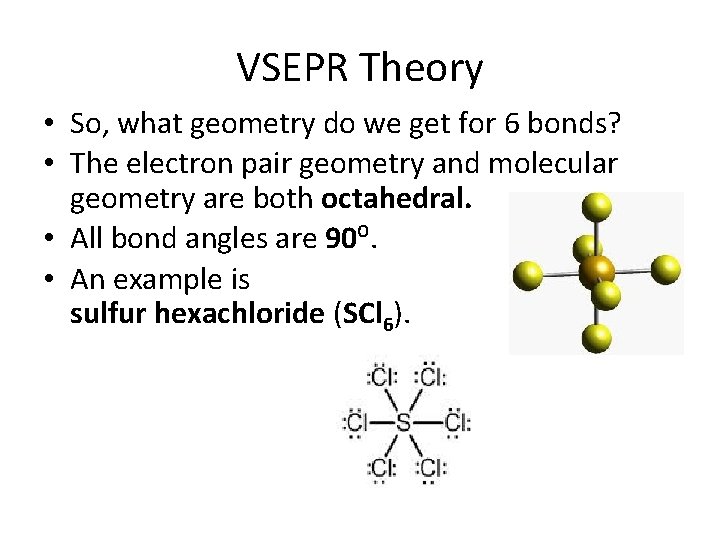
VSEPR Theory • So, what geometry do we get for 6 bonds? • The electron pair geometry and molecular geometry are both octahedral. • All bond angles are 90⁰. • An example is sulfur hexachloride (SCl 6).

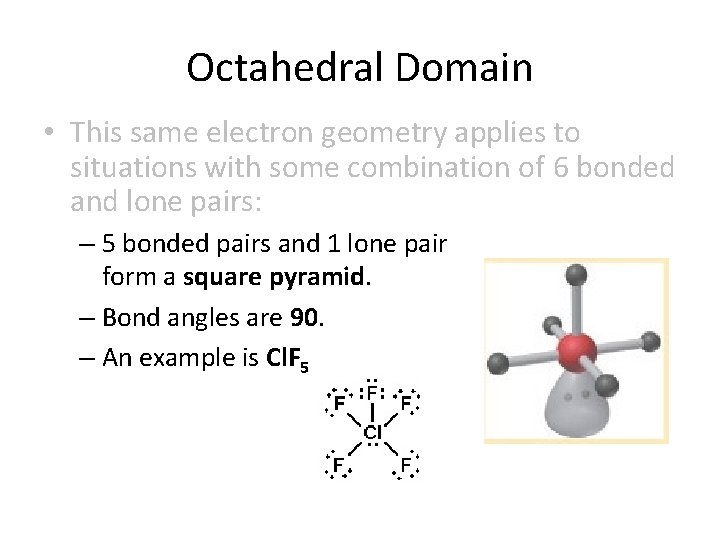
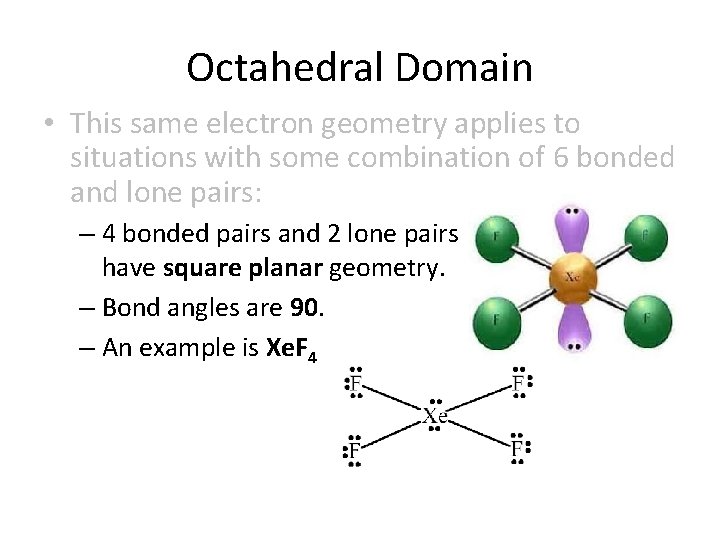
Octahedral Domain • This same electron geometry applies to situations with some combination of 6 bonded and lone pairs: – 5 bonded pairs and 1 lone pair form a square pyramid. – Bond angles are 90. – An example is Cl. F 5

Octahedral Domain • This same electron geometry applies to situations with some combination of 6 bonded and lone pairs: – 4 bonded pairs and 2 lone pairs have square planar geometry. – Bond angles are 90. – An example is Xe. F 4

VSEPR Theory • Lastly, double and triple bonds are treated in the same way as single bonds. • Polyatomic ions are treated the same way that molecules are.

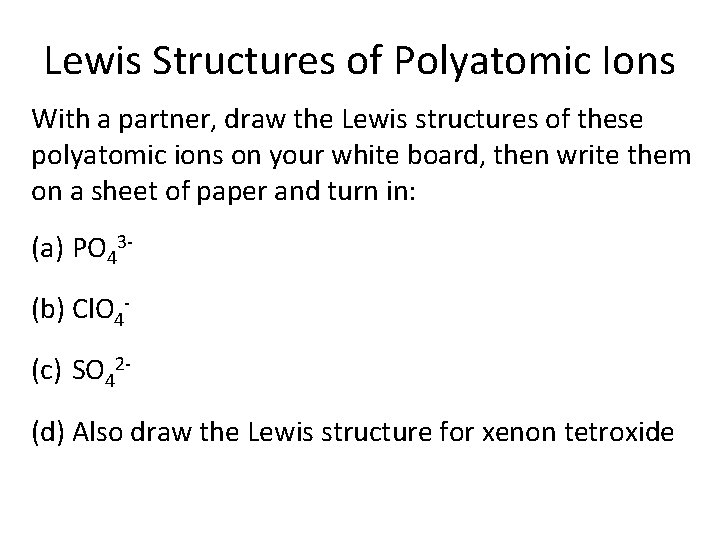
Lewis Structures of Polyatomic Ions With a partner, draw the Lewis structures of these polyatomic ions on your white board, then write them on a sheet of paper and turn in: (a) PO 43(b) Cl. O 4(c) SO 42(d) Also draw the Lewis structure for xenon tetroxide

Intermolecular Forces • The forces of attraction between molecules are known as intermolecular forces. While they vary in strength, they are generally weaker than the bonds that join atoms in molecules, ions in ionic compounds, or metal atoms in solid metals. • A compound’s boiling point is a good measure of the force of attraction between particles of a substance when it is a liquid. Substances with ionic and metallic bonds have much higher boiling points than substances with covalent bonds.

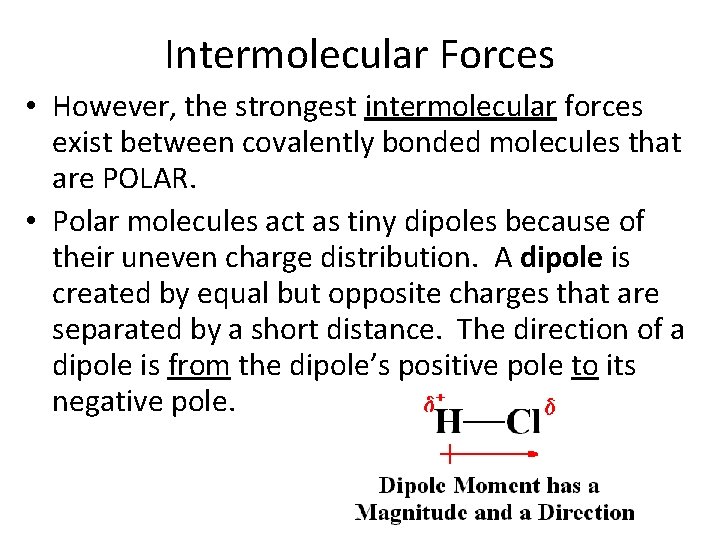
Intermolecular Forces • However, the strongest intermolecular forces exist between covalently bonded molecules that are POLAR. • Polar molecules act as tiny dipoles because of their uneven charge distribution. A dipole is created by equal but opposite charges that are separated by a short distance. The direction of a dipole is from the dipole’s positive pole to its negative pole.

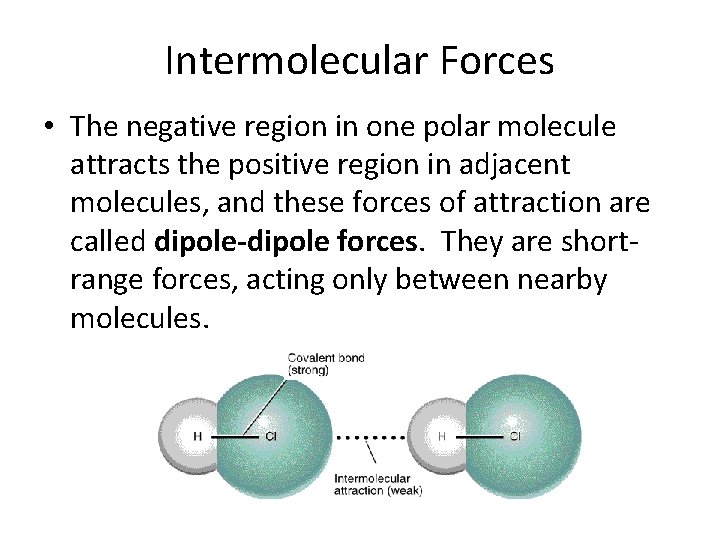
Intermolecular Forces • The negative region in one polar molecule attracts the positive region in adjacent molecules, and these forces of attraction are called dipole-dipole forces. They are shortrange forces, acting only between nearby molecules.

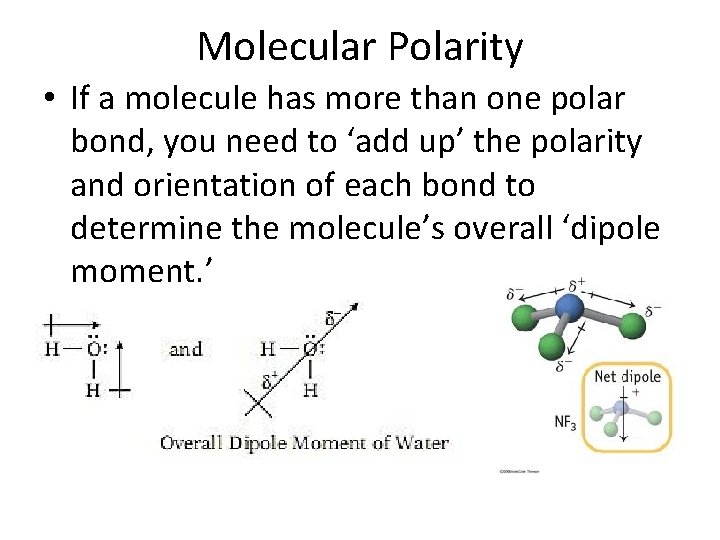
Molecular Polarity • If a molecule has more than one polar bond, you need to ‘add up’ the polarity and orientation of each bond to determine the molecule’s overall ‘dipole moment. ’

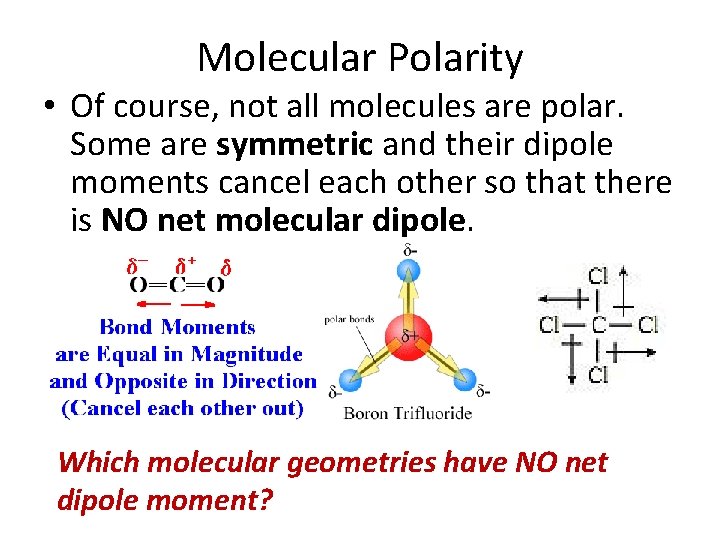
Molecular Polarity • Of course, not all molecules are polar. Some are symmetric and their dipole moments cancel each other so that there is NO net molecular dipole. Which molecular geometries have NO net dipole moment?

Molecular Polarity • Now let’s go back through your VSEPR and Molecular Geometry chart and label each type of molecule as ‘polar’ (P) or ‘non-polar’ (NP) by its geometry. • The polar geometries are: Linear: v-shaped Tetrahedral: trigonal pyramidal, v-shaped Trigonal bipyramidal: see-saw, T-structure (shaped) and linear Octahedral: Square pyramid

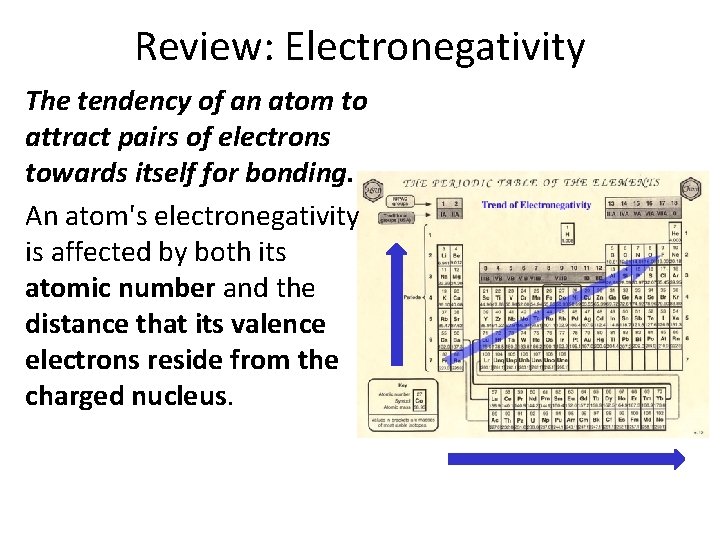
Review: Electronegativity The tendency of an atom to attract pairs of electrons towards itself for bonding. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus.

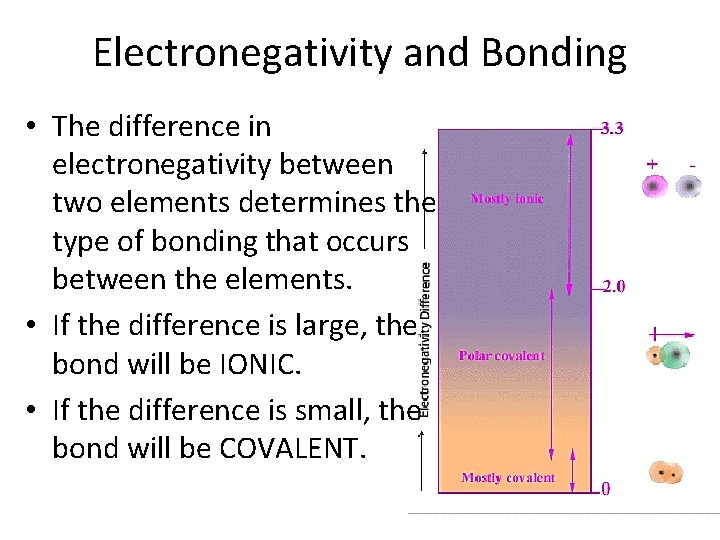
Electronegativity and Bonding • The difference in electronegativity between two elements determines the type of bonding that occurs between the elements. • If the difference is large, the bond will be IONIC. • If the difference is small, the bond will be COVALENT.

Electronegativity • Moving left to right across a period, electronegativity increases due to the stronger attraction for electrons as the nuclear charge increases. • Moving down a group, electronegativity decreases due to the longer distance between the nucleus and the valence electron shell, reducing the attraction for electrons.

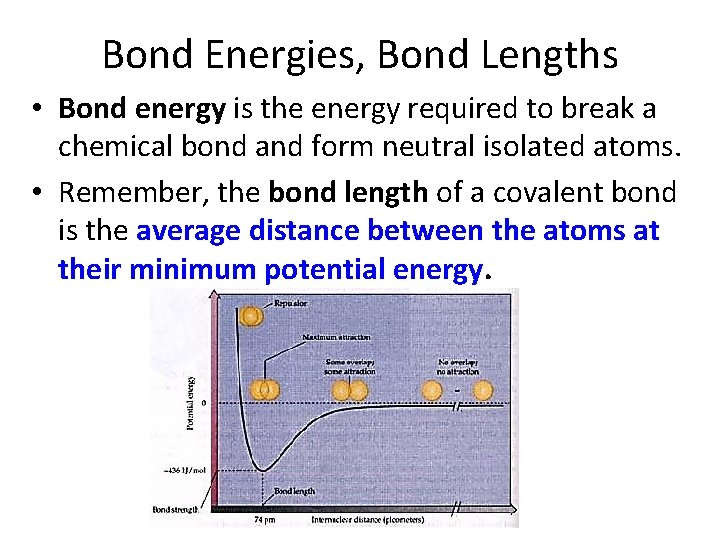
Bond Energies, Bond Lengths • Bond energy is the energy required to break a chemical bond and form neutral isolated atoms. • Remember, the bond length of a covalent bond is the average distance between the atoms at their minimum potential energy.

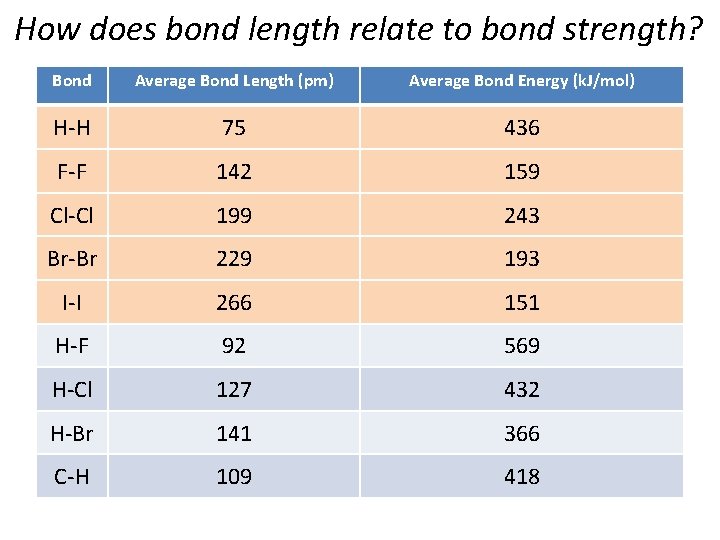
How does bond length relate to bond strength? Bond Average Bond Length (pm) Average Bond Energy (k. J/mol) H-H 75 436 F-F 142 159 Cl-Cl 199 243 Br-Br 229 193 I-I 266 151 H-F 92 569 H-Cl 127 432 H-Br 141 366 C-H 109 418

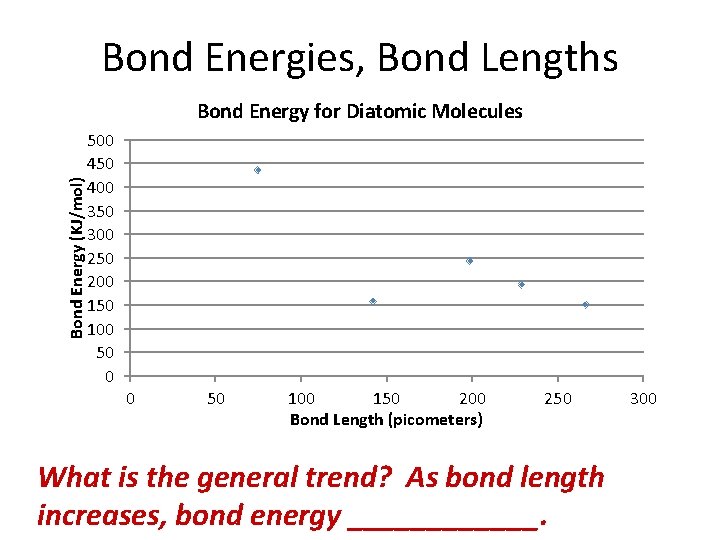
Bond Energies, Bond Lengths Bond Energy (KJ/mol) Bond Energy for Diatomic Molecules 500 450 400 350 300 250 200 150 100 50 0 0 50 100 150 200 Bond Length (picometers) 250 What is the general trend? As bond length increases, bond energy ______. 300

Demonstrations • We need two boys to demonstrate types and lengths of bonds. Who will volunteer? • Now we need a girl to help the boys demonstrate bond strength.

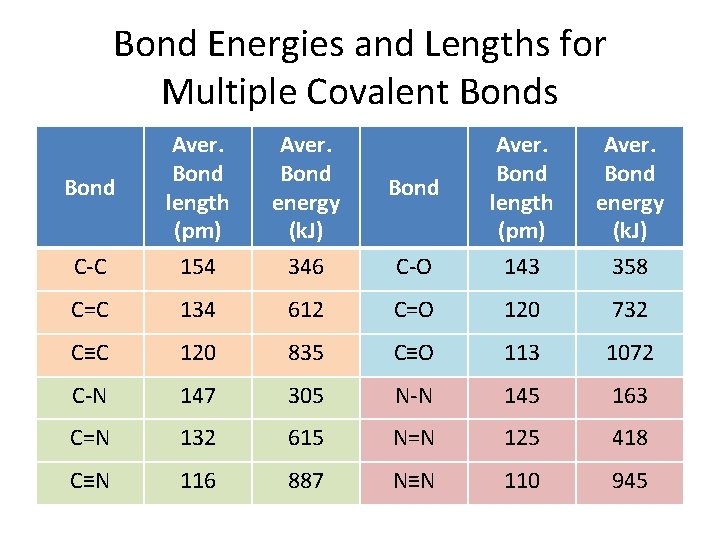
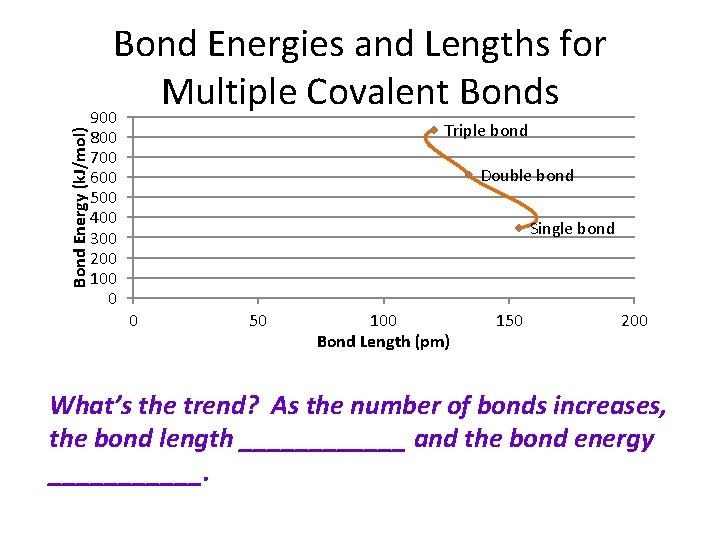
Bond Energies and Lengths for Multiple Covalent Bonds C-C Aver. Bond length (pm) 154 Aver. Bond energy (k. J) 346 C-O Aver. Bond length (pm) 143 Aver. Bond energy (k. J) 358 C=C 134 612 C=O 120 732 C≡C 120 835 C≡O 113 1072 C-N 147 305 N-N 145 163 C=N 132 615 N=N 125 418 C≡N 116 887 N≡N 110 945 Bond

Bond Energy (k. J/mol) Bond Energies and Lengths for Multiple Covalent Bonds 900 800 700 600 500 400 300 200 100 0 Triple bond Double bond Single bond 0 50 100 Bond Length (pm) 150 200 What’s the trend? As the number of bonds increases, the bond length ______ and the bond energy ______.

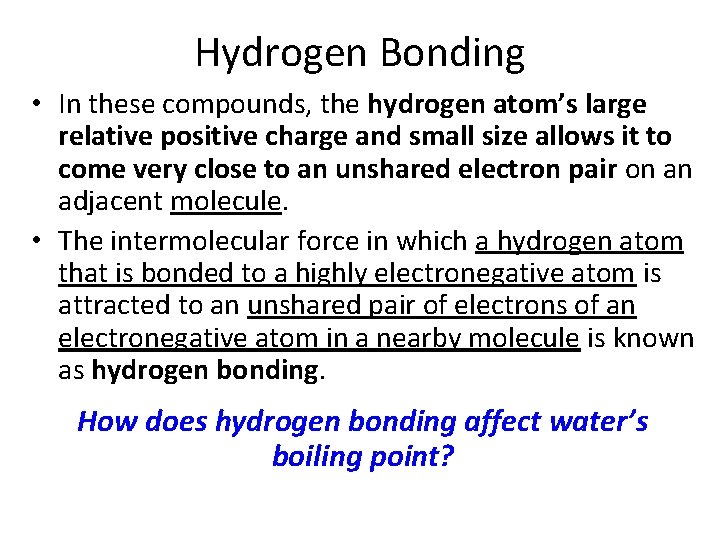
Hydrogen Bonding • Some hydrogen-containing compounds, such as hydrogen fluoride (HF), water (H 2 O), and ammonia (NH 3) have unusually high boiling points. • This is explained by a particularly strong type of dipole-dipole force. The large electronegativity differences between hydrogen and fluorine, oxygen, and nitrogen atoms makes their bonds highly polar.

Hydrogen Bonding • In these compounds, the hydrogen atom’s large relative positive charge and small size allows it to come very close to an unshared electron pair on an adjacent molecule. • The intermolecular force in which a hydrogen atom that is bonded to a highly electronegative atom is attracted to an unshared pair of electrons of an electronegative atom in a nearby molecule is known as hydrogen bonding. How does hydrogen bonding affect water’s boiling point?

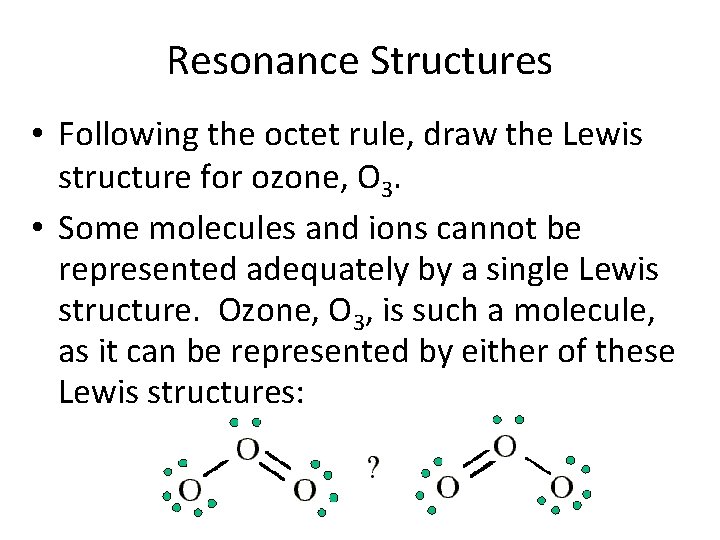
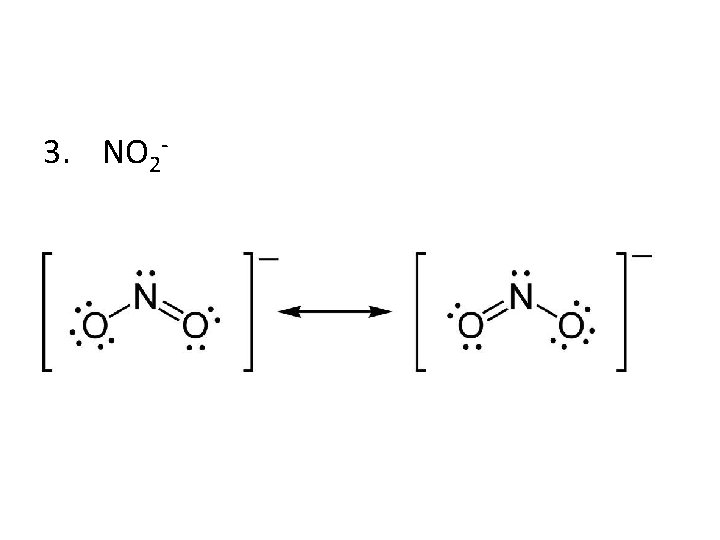
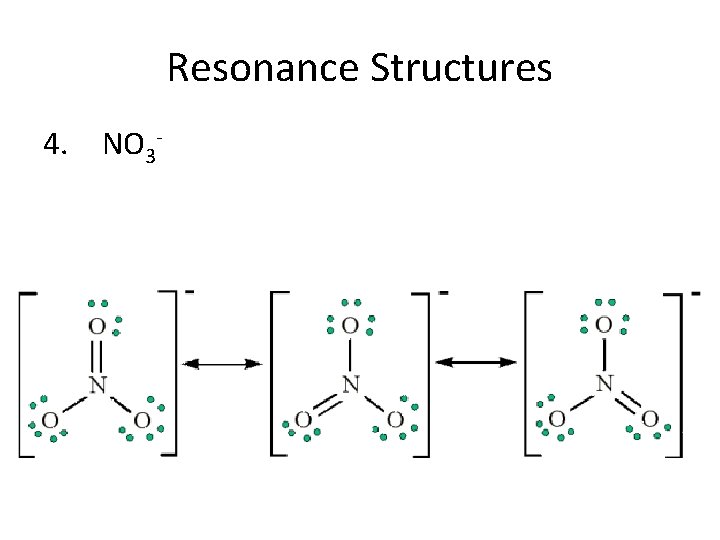
Resonance Structures • Following the octet rule, draw the Lewis structure for ozone, O 3. • Some molecules and ions cannot be represented adequately by a single Lewis structure. Ozone, O 3, is such a molecule, as it can be represented by either of these Lewis structures:

Resonance Structures • Scientists used to believe that ozone spent its time constantly alternating or ‘resonating’ between these two structures. • Experiments have shown, however, that the oxygen-oxygen bonds in ozone are identical, not sometimes a single and sometimes a double bond. Chemists now say that ozone has a structure that is the average of these two. Together the structures are called resonance structures or resonance hybrids. • Resonance refers to bonding in molecules that cannot be correctly represented by a single Lewis structure.

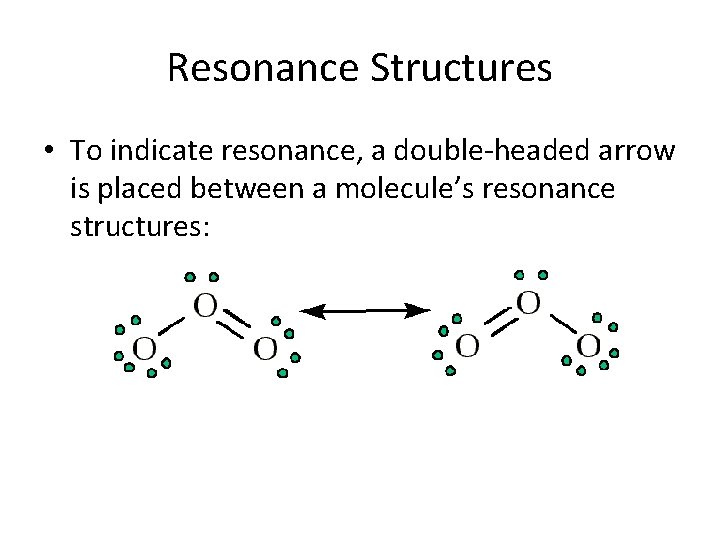
Resonance Structures • To indicate resonance, a double-headed arrow is placed between a molecule’s resonance structures:

Resonance Structures Write Lewis structures for each of the following molecules. Remember to (1) sum up the valence electrons, (2) put in single bonds, and (3) satisfy the octet rule. 1. CO 2 2. CO 323. NO 24. NO 3 -

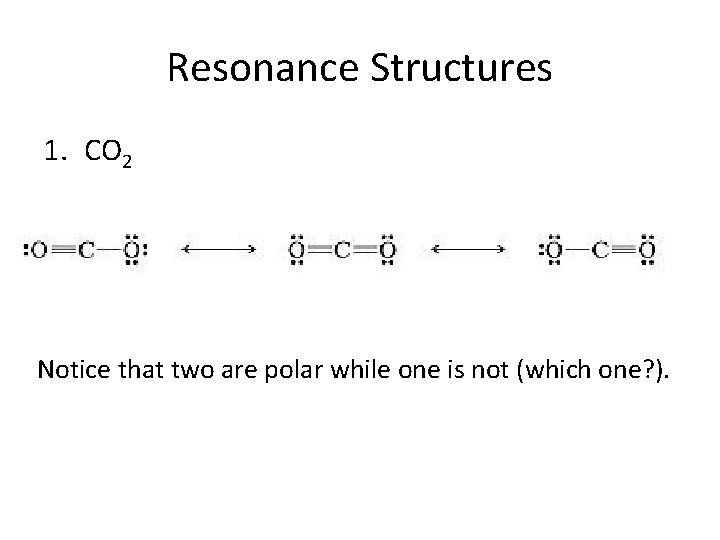
Resonance Structures 1. CO 2 Notice that two are polar while one is not (which one? ).

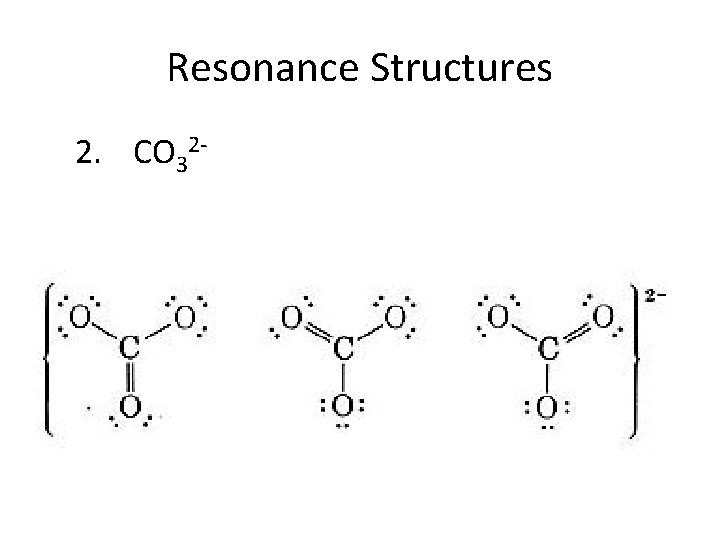
Resonance Structures 2. CO 32 -

3. NO 2 -

Resonance Structures 4. NO 3 -