Properties of Water Polar molecule Cohesion and adhesion














![Problem How much greater is the [ H+ ] in a solution with p. Problem How much greater is the [ H+ ] in a solution with p.](https://slidetodoc.com/presentation_image/2bf142f7fc559df275afe3abbd831e7b/image-27.jpg)


- Slides: 29

Properties of Water • Polar molecule • Cohesion and adhesion • High specific heat • Density – greatest at 4 o. C • Universal solvent of life

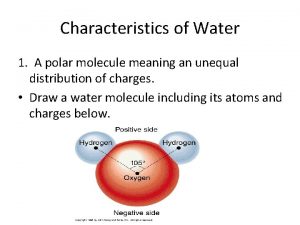
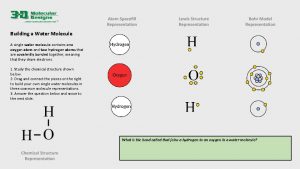
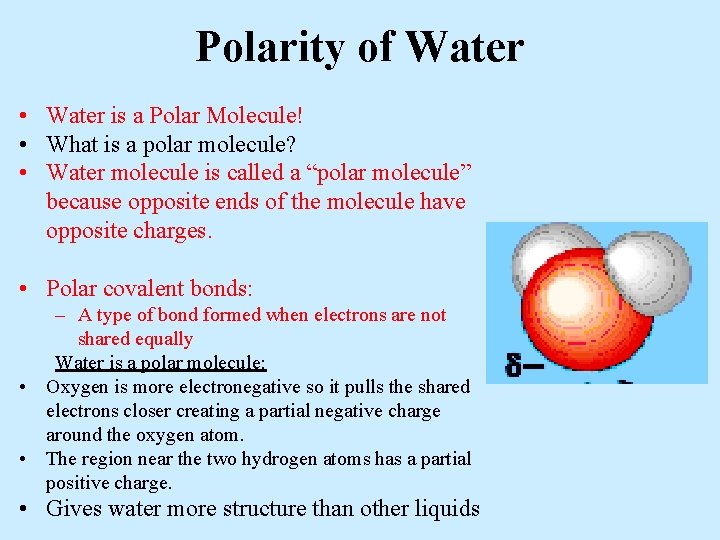
Polarity of Water • Water is a Polar Molecule! • What is a polar molecule? • Water molecule is called a “polar molecule” because opposite ends of the molecule have opposite charges. • Polar covalent bonds: – A type of bond formed when electrons are not shared equally Water is a polar molecule: • Oxygen is more electronegative so it pulls the shared electrons closer creating a partial negative charge around the oxygen atom. • The region near the two hydrogen atoms has a partial positive charge. • Gives water more structure than other liquids

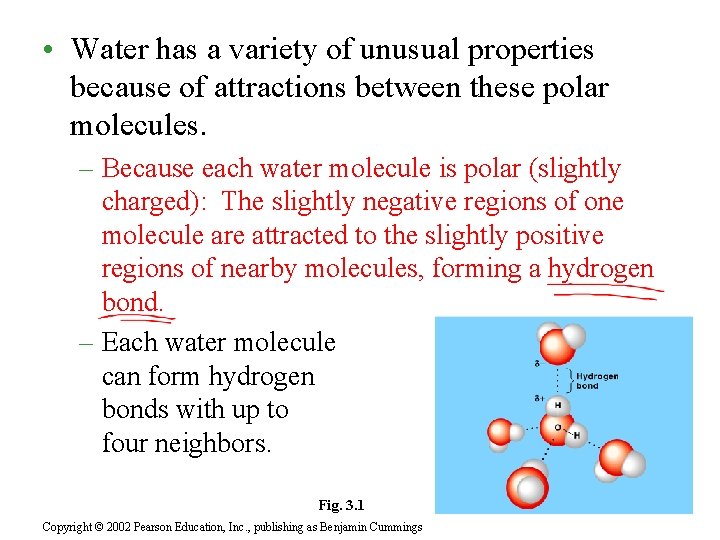
• Water has a variety of unusual properties because of attractions between these polar molecules. – Because each water molecule is polar (slightly charged): The slightly negative regions of one molecule are attracted to the slightly positive regions of nearby molecules, forming a hydrogen bond. – Each water molecule can form hydrogen bonds with up to four neighbors. Fig. 3. 1 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

HYDROGEN BONDS give water the following properties: • Hold water molecules together • Each water molecule can form a maximum of 4 hydrogen bonds • The hydrogen bonds joining water molecules are weak, about 1/20 th as strong as covalent bonds. • They form, break, and reform with great frequency • Extraordinary Properties that are a result of hydrogen bonds. – – – Cohesive behavior Resists changes in temperature High heat of vaporization Expands when it freezes Versatile solvent

Organisms Depend on Cohesion Hydrogen bonds hold the substance together, a phenomenon called cohesion • Cohesion: the act of sticking together – example: water clinging to other water molecules • Cohesion among water molecules plays a key role in the transport of water against gravity in plants • Adhesion: clinging of one substance to another – Ex. water clings to the sides of a vessel

Let’s talk! Cohesion vs. adhesion Cohesion is different from adhesion because…… When you have an answer, put your pencil down and nod.

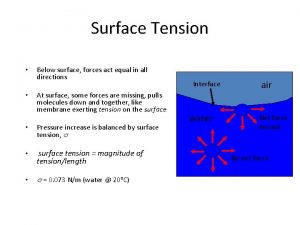
• Water has a high surface tension (a measure of the force required to break the surface of a liquid) because the molecules are cohesive. – Water has a greater surface tension than most other liquids because hydrogen bonds among surface water molecules resist stretching or breaking the surface. – Water behaves as if covered by an invisible film. – Some animals can stand, walk, or run on water without breaking the surface. • A surfactant (wetting agent) will reduce the surface tension of water. Soap is an example of a surfactant. Fig. 3. 3 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Video! • Surface Tension and Capillary Action Video

Specific Heat is the amount of heat that must be absorbed or lost for one gram of a substance to change its temperature by 1 o. C. Three-fourths of the earth is covered by water. The water serves as a large heat sink responsible for: 1. Prevention of temperature fluctuations that are outside the range suitable for life. 2. Coastal areas having a mild climate 3. A stable marine environment

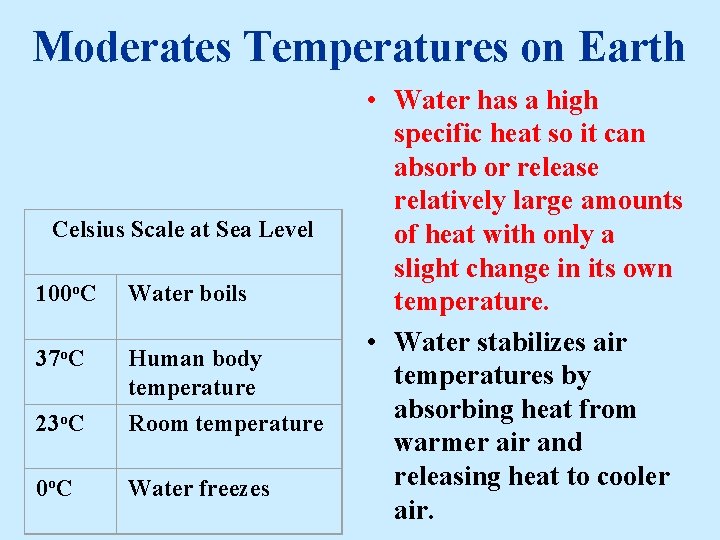
Moderates Temperatures on Earth Celsius Scale at Sea Level 100 o. C Water boils 37 o. C 23 o. C Human body temperature Room temperature 0 o. C Water freezes • Water has a high specific heat so it can absorb or release relatively large amounts of heat with only a slight change in its own temperature. • Water stabilizes air temperatures by absorbing heat from warmer air and releasing heat to cooler air.

Evaporative Cooling • The cooling of a surface occurs when the liquid evaporates • This is responsible for: – Moderating earth’s climate – Stabilizes temperature in aquatic ecosystems – Preventing organisms from overheating

Density of Water • Most dense at 4 o. C • Contracts until 4 o. C • Expands from 4 o. C to 0 o. C The density of water: 1. Ice is less dense than water so ice floats. 2. Prevents lakes from freezing from the bottom up. 3. Ice forms on the surface first—the freezing of the water releases heat to the water below creating insulation. 1. Makes transition between season less abrupt.

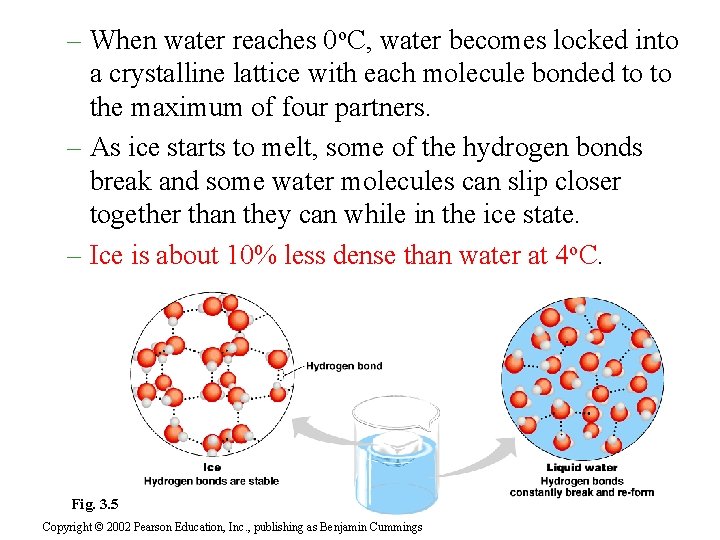
– When water reaches 0 o. C, water becomes locked into a crystalline lattice with each molecule bonded to to the maximum of four partners. – As ice starts to melt, some of the hydrogen bonds break and some water molecules can slip closer together than they can while in the ice state. – Ice is about 10% less dense than water at 4 o. C. Fig. 3. 5 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Video! • Water Freezing at Molecular Level

Its just the tip of the iceberg…

Water is the Universal Solvent • Water will dissolve: – Ionic compounds (salt) – Polar molecules – “like dissolves like” • Water will not dissolve: – Nonpolar compounds • Ex. oil

Word Pictures Game • Get into lab groups. • Form 2 teams within each group. One team on each side of the table. • Select one of the words from a card and illustrate it on the board. • The opposing team gets a point for a correct guess. • The other team illustrates another word.

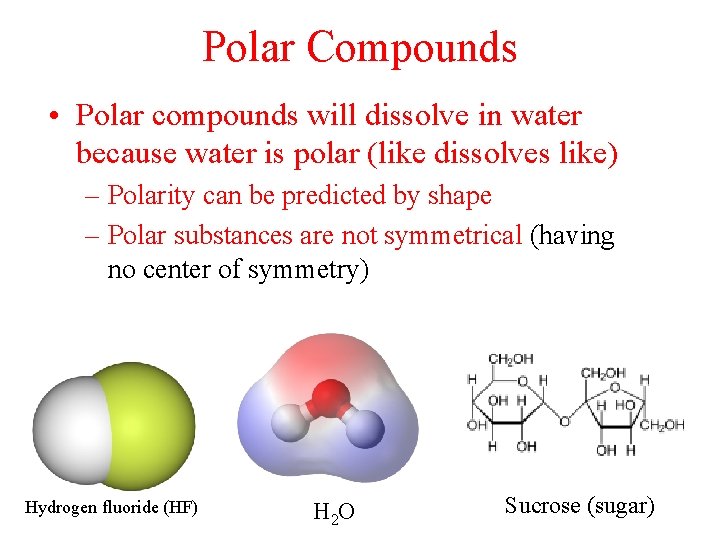
Polar Compounds • Polar compounds will dissolve in water because water is polar (like dissolves like) – Polarity can be predicted by shape – Polar substances are not symmetrical (having no center of symmetry) Hydrogen fluoride (HF) H 2 O Sucrose (sugar)

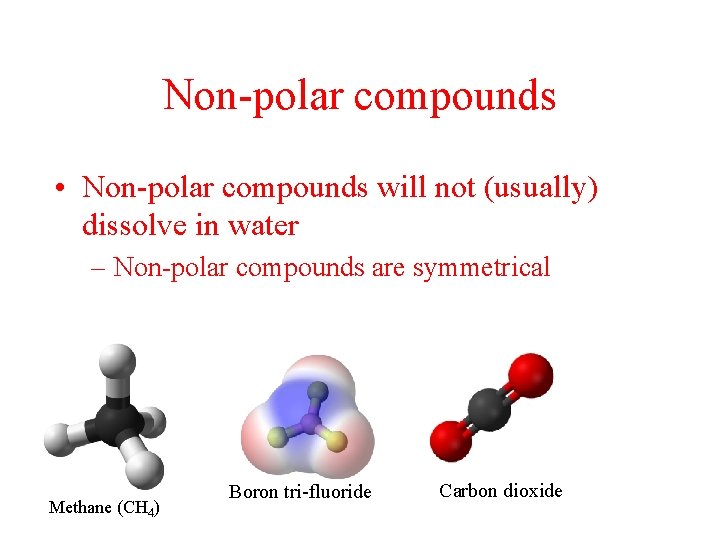
Non-polar compounds • Non-polar compounds will not (usually) dissolve in water – Non-polar compounds are symmetrical Methane (CH 4) Boron tri-fluoride Carbon dioxide

Most biochemical reactions involve solutes dissolved in water. • There are two important quantitative proprieties of aqueous solutions. – 1. Concentration – 2. p. H

Concentration of a Solution • Molecular weight – sum of the weights of all atoms in a molecule (daltons) • Mole – amount of a substance that has a mass in grams numerically equivalent to its molecular weight in daltons. • Avogadro’s number – 6. 02 X 1023 – A mole of one substance has the same number of molecules as a mole of any other substance.

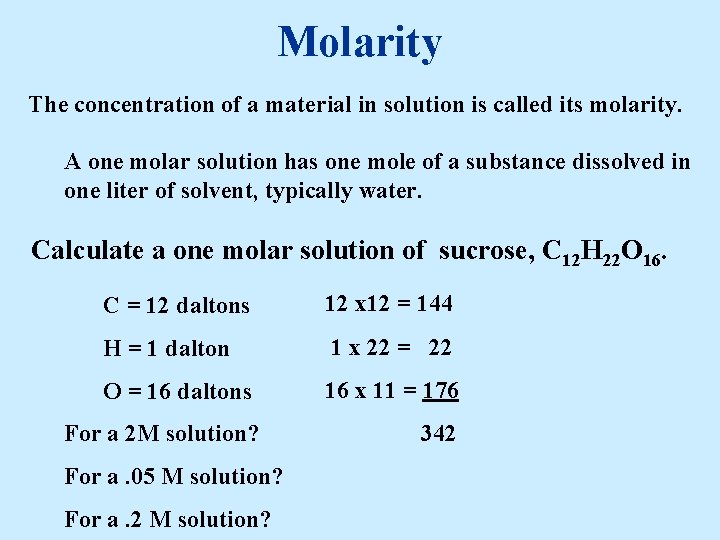
Molarity The concentration of a material in solution is called its molarity. A one molar solution has one mole of a substance dissolved in one liter of solvent, typically water. Calculate a one molar solution of sucrose, C 12 H 22 O 16. C = 12 daltons 12 x 12 = 144 H = 1 dalton 1 x 22 = 22 O = 16 daltons 16 x 11 = 176 For a 2 M solution? For a. 05 M solution? For a. 2 M solution? 342

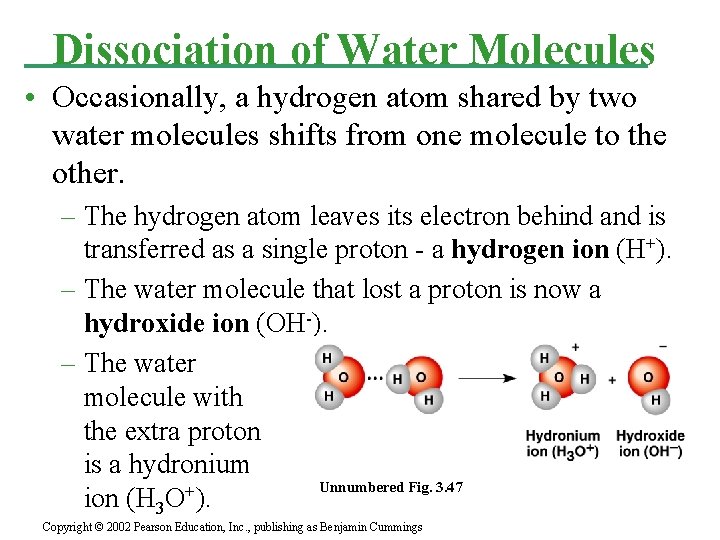
Dissociation of Water Molecules • Occasionally, a hydrogen atom shared by two water molecules shifts from one molecule to the other. – The hydrogen atom leaves its electron behind and is transferred as a single proton - a hydrogen ion (H+). – The water molecule that lost a proton is now a hydroxide ion (OH-). – The water molecule with the extra proton is a hydronium Unnumbered Fig. 3. 47 ion (H 3 O+). Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

• A simpler way to view this process is that a water molecule dissociates into a hydrogen ion and a hydroxide ion: – H 2 O <=> H+ + OH • This reaction is reversible. • At equilibrium the concentration of water molecules greatly exceeds that of H+ and OH-. • In pure water only one water molecule in every 554 million is dissociated. – At equilibrium, the concentration of H+ or OH- is 10 -7 M (25°C).

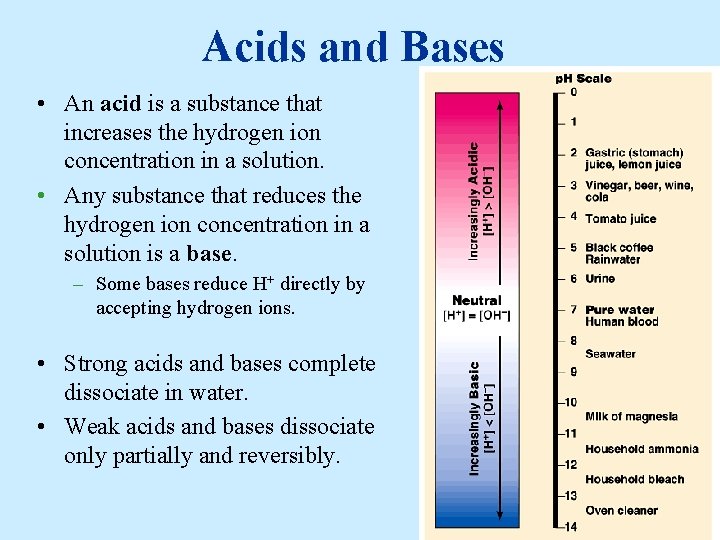
Acids and Bases • An acid is a substance that increases the hydrogen ion concentration in a solution. • Any substance that reduces the hydrogen ion concentration in a solution is a base. – Some bases reduce H+ directly by accepting hydrogen ions. • Strong acids and bases complete dissociate in water. • Weak acids and bases dissociate only partially and reversibly.

p. H Scale • The p. H scale in any aqueous solution : – [ H+ ] [OH-] = 10 -14 • Measures the degree of acidity (0 – 14) • Most biologic fluids are in the p. H range from 6 – 8 • Each p. H unit represents a tenfold difference (scale is logarithmic) – A small change in p. H actually indicates a substantial change in H+ and OH- concentrations.
![Problem How much greater is the H in a solution with p Problem How much greater is the [ H+ ] in a solution with p.](https://slidetodoc.com/presentation_image/2bf142f7fc559df275afe3abbd831e7b/image-27.jpg)
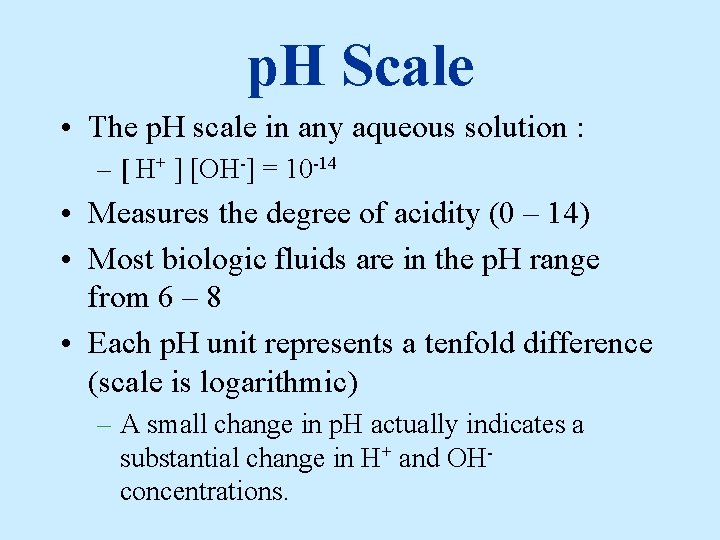
Problem How much greater is the [ H+ ] in a solution with p. H 2 than in a solution with p. H 6? Answer: p. H of 2 = [ H+ ] of 1. 0 x 10 -2 = 1/100 M p. H of 6 = [ H+ ] of 1. 0 x 10 -6 = 1/1, 000 M 10, 000 times greater

Buffers • A substance that eliminates large sudden changes in p. H. • Buffers help organisms maintain the p. H of body fluids within the narrow range necessary for life. – Are combinations of H+ acceptors and donors forms in a solution of weak acids or bases – Work by accepting H+ from solutions when they are in excess and by donating H+ when they have been depleted.

Acid Precipitation • Rain, snow or fog with more strongly acidic than p. H of 5. 6 • West Virginia has recorded 1. 5 • East Tennessee reported 4. 2 in 2000 • Occurs when sulfur oxides and nitrogen oxides react with water in the atmosphere – Lowers p. H of soil which affects mineral solubility – decline of forests – Lower p. H of lakes and ponds – In the Western Adirondack Mountains, there are lakes with a p. H <5 that have no fish.
 Adhesive and cohesive forces
Adhesive and cohesive forces Polar molecule
Polar molecule Adhesion cohesion
Adhesion cohesion Adhesion cohesion
Adhesion cohesion Adhesion cohesion
Adhesion cohesion Water potential in transpiration
Water potential in transpiration Cohesion or adhesion
Cohesion or adhesion Polar molecule meaning
Polar molecule meaning Watercovalent bond
Watercovalent bond Polar covalent bond in water molecule
Polar covalent bond in water molecule Polar covalent bond
Polar covalent bond Water and water and water water
Water and water and water water What is adhesion in water
What is adhesion in water Properties of water hydrogen bonding
Properties of water hydrogen bonding Is h2o a polar molecule
Is h2o a polar molecule Nh3 shape and polarity
Nh3 shape and polarity Fffpf
Fffpf Hydration shells
Hydration shells Polar molecule
Polar molecule Polar molecule
Polar molecule Polar molecule
Polar molecule Polar molecule examples
Polar molecule examples Polar and nonpolar
Polar and nonpolar Polar and non polar amino acids
Polar and non polar amino acids What are polar and nonpolar dielectrics
What are polar and nonpolar dielectrics Sustancia polar y no polar
Sustancia polar y no polar Polar polar attractions
Polar polar attractions Qumica
Qumica Medios hipertonicos
Medios hipertonicos Mechanical interlocking adhesion
Mechanical interlocking adhesion