Section 5 4Polarity of Molecules Electronegativity l Definition


- Slides: 17

Section 5. 4—Polarity of Molecules

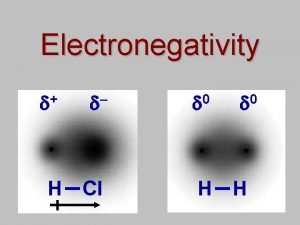
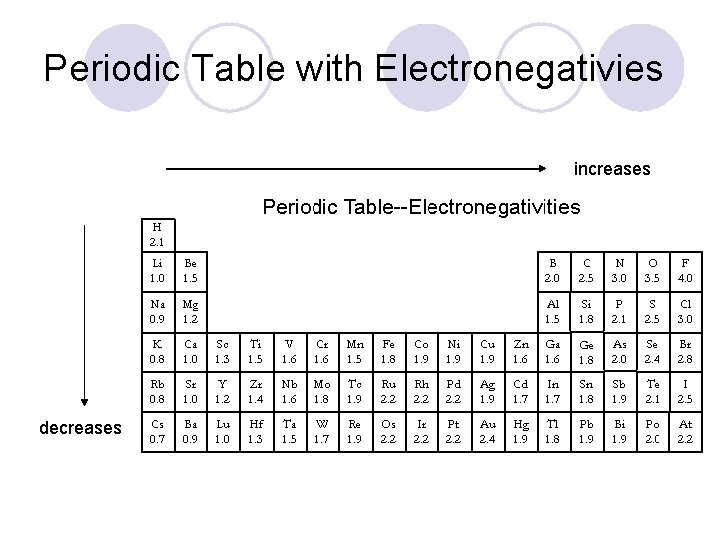
Electronegativity l Definition: The pull an atom has for the electrons it shares with another atom in a bond. l Electronegativity is a periodic trend ¡As atomic radius increases and number of electron shells increases, the nucleus of an atom has less of a pull on its outermost electrons and the electrons within a bond!

Periodic Table with Electronegativies increases decreases

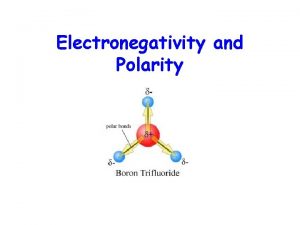
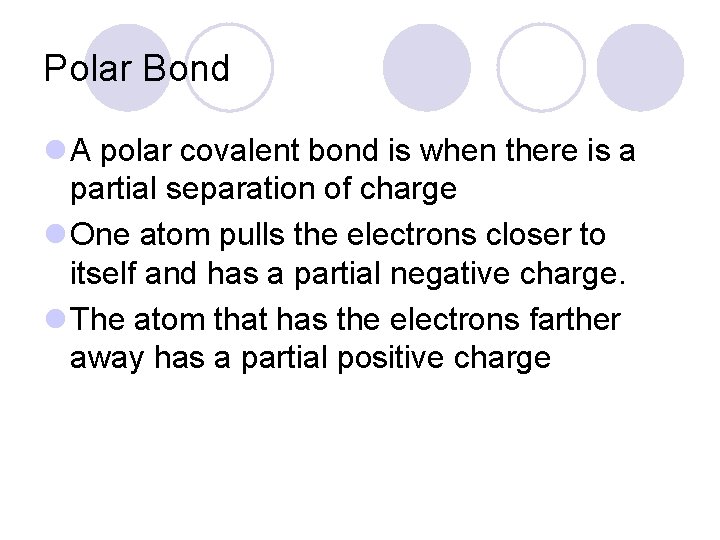
Polar Bond l A polar covalent bond is when there is a partial separation of charge l One atom pulls the electrons closer to itself and has a partial negative charge. l The atom that has the electrons farther away has a partial positive charge

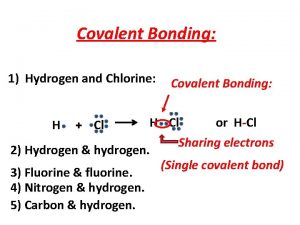
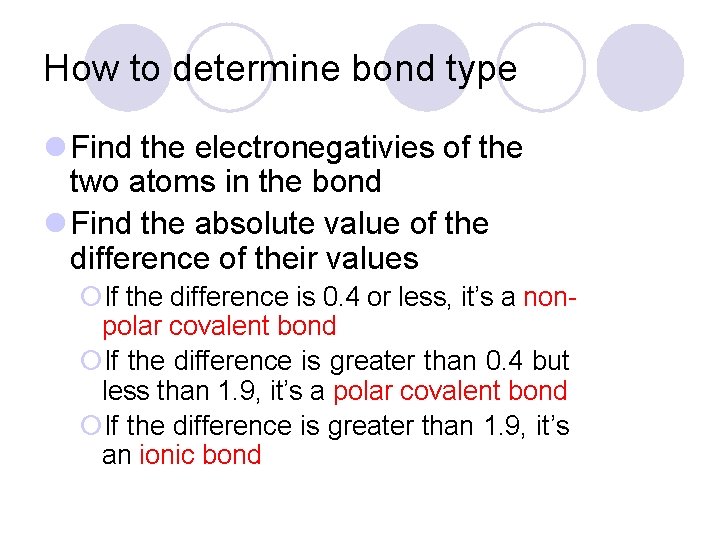
How to determine bond type l Find the electronegativies of the two atoms in the bond l Find the absolute value of the difference of their values ¡If the difference is 0. 4 or less, it’s a nonpolar covalent bond ¡If the difference is greater than 0. 4 but less than 1. 9, it’s a polar covalent bond ¡If the difference is greater than 1. 9, it’s an ionic bond

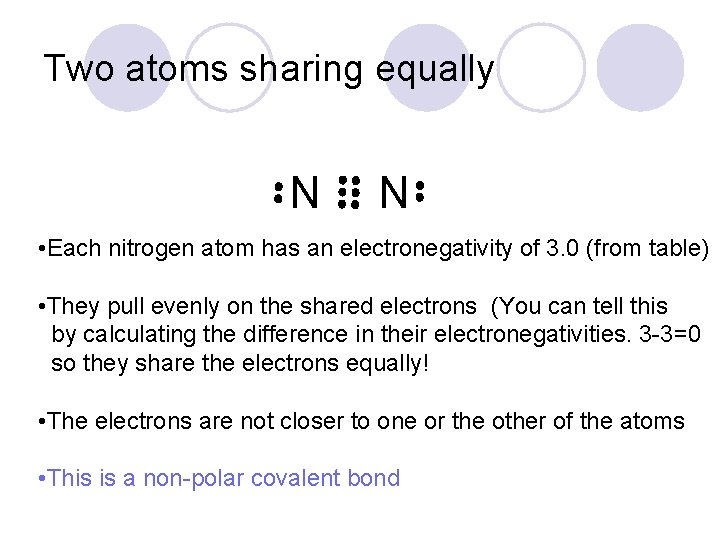
Two atoms sharing equally N N • Each nitrogen atom has an electronegativity of 3. 0 (from table) • They pull evenly on the shared electrons (You can tell this by calculating the difference in their electronegativities. 3 -3=0 so they share the electrons equally! • The electrons are not closer to one or the other of the atoms • This is a non-polar covalent bond

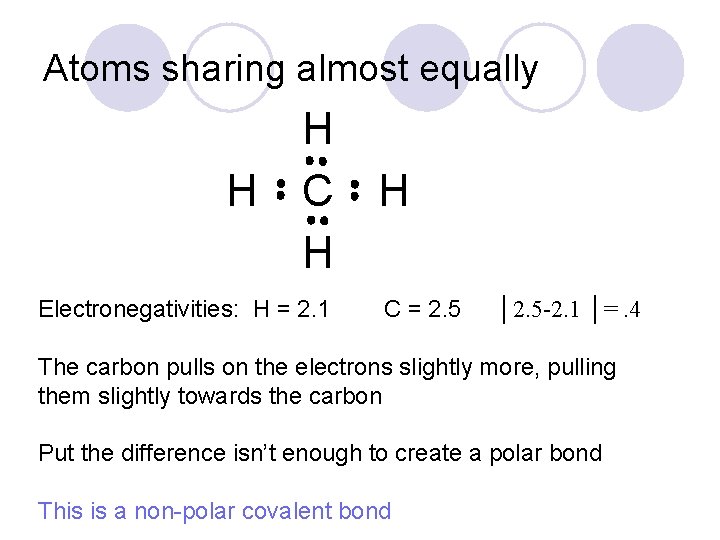
Atoms sharing almost equally H H C H Electronegativities: H = 2. 1 H C = 2. 5 │2. 5 -2. 1 │=. 4 The carbon pulls on the electrons slightly more, pulling them slightly towards the carbon Put the difference isn’t enough to create a polar bond This is a non-polar covalent bond

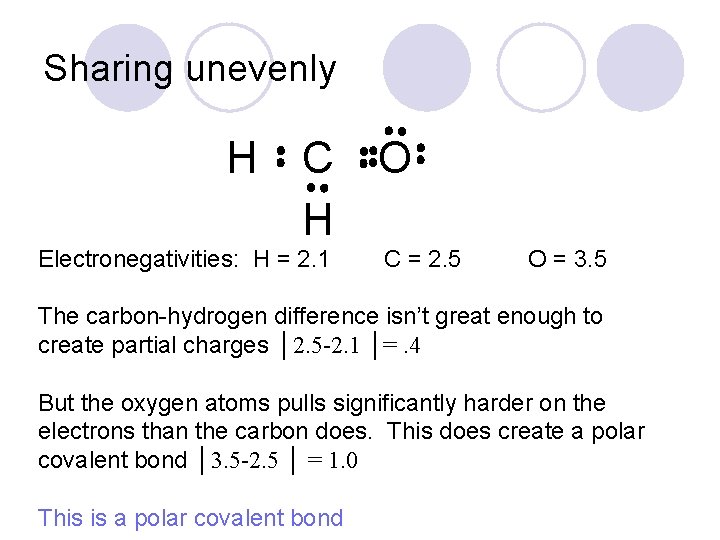
Sharing unevenly H C H Electronegativities: H = 2. 1 O C = 2. 5 O = 3. 5 The carbon-hydrogen difference isn’t great enough to create partial charges │2. 5 -2. 1 │=. 4 But the oxygen atoms pulls significantly harder on the electrons than the carbon does. This does create a polar covalent bond │3. 5 -2. 5 │ = 1. 0 This is a polar covalent bond

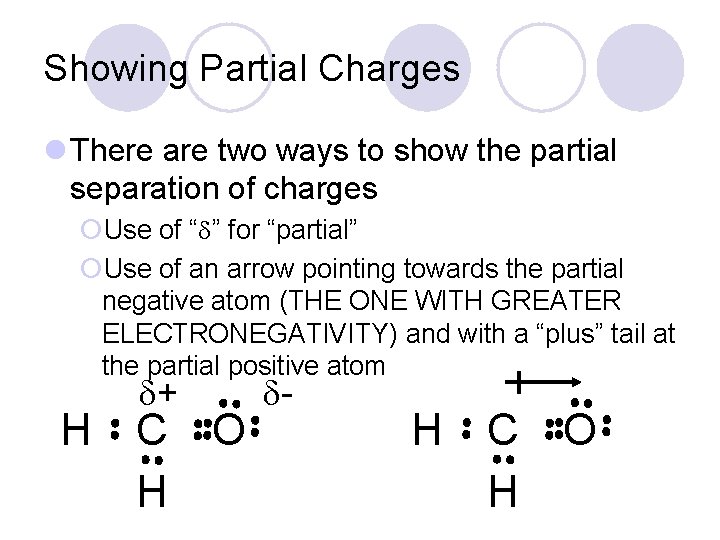
Showing Partial Charges l There are two ways to show the partial separation of charges ¡Use of “ ” for “partial” ¡Use of an arrow pointing towards the partial negative atom (THE ONE WITH GREATER ELECTRONEGATIVITY) and with a “plus” tail at the partial positive atom H + C H O - H C H O

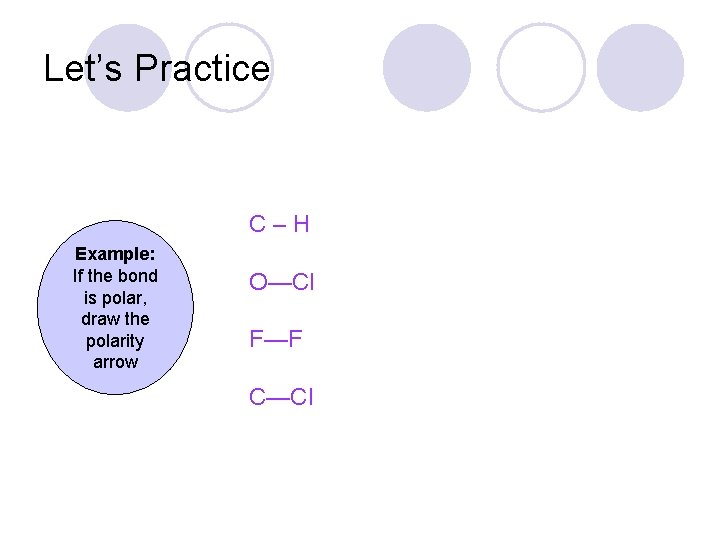
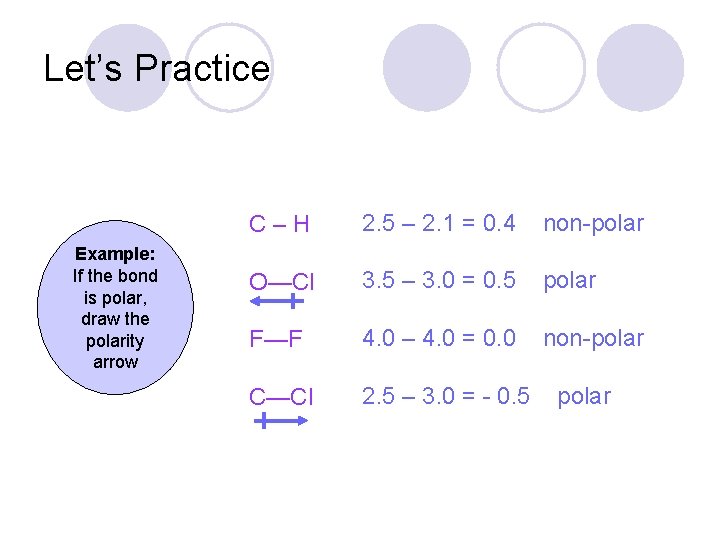
Let’s Practice C–H Example: If the bond is polar, draw the polarity arrow O—Cl F—F C—Cl

Let’s Practice Example: If the bond is polar, draw the polarity arrow C–H 2. 5 – 2. 1 = 0. 4 non-polar O—Cl 3. 5 – 3. 0 = 0. 5 polar F—F 4. 0 – 4. 0 = 0. 0 non-polar C—Cl 2. 5 – 3. 0 = - 0. 5 polar

Ionic Bonds l Ionic bonds occur when the electronegativies of two atoms are so different that they can’t even share unevenly…one atom just takes them from the other

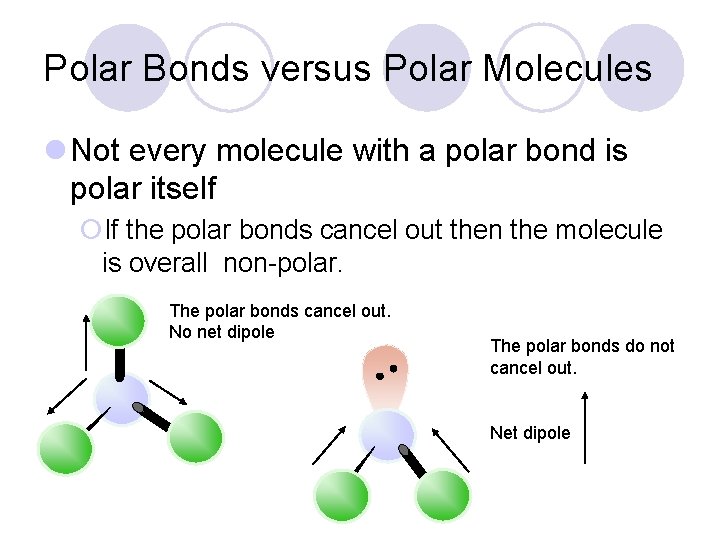
Polar Bonds versus Polar Molecules l Not every molecule with a polar bond is polar itself ¡If the polar bonds cancel out then the molecule is overall non-polar. The polar bonds cancel out. No net dipole The polar bonds do not cancel out. Net dipole

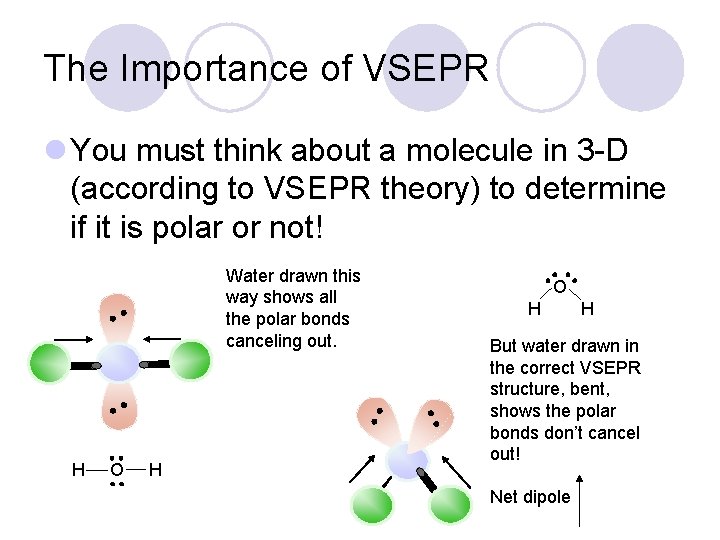
The Importance of VSEPR l You must think about a molecule in 3 -D (according to VSEPR theory) to determine if it is polar or not! Water drawn this way shows all the polar bonds canceling out. H O H H But water drawn in the correct VSEPR structure, bent, shows the polar bonds don’t cancel out! Net dipole

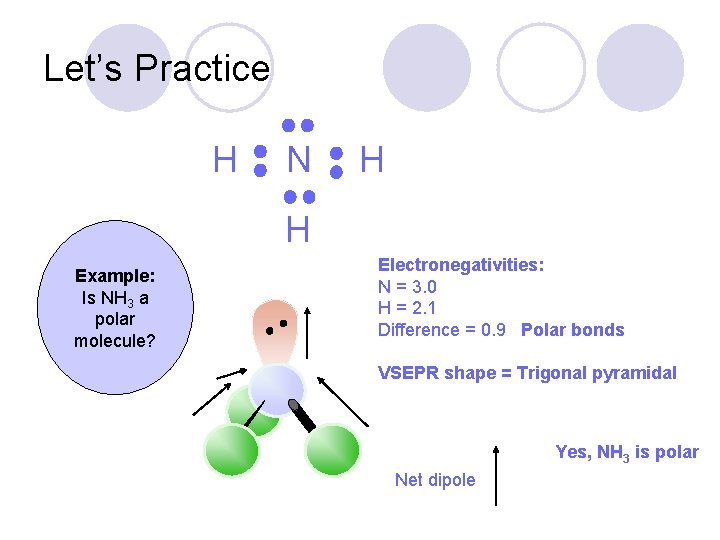
Let’s Practice Example: Is NH 3 a polar molecule?

Let’s Practice H N H H Example: Is NH 3 a polar molecule? Electronegativities: N = 3. 0 H = 2. 1 Difference = 0. 9 Polar bonds VSEPR shape = Trigonal pyramidal Yes, NH 3 is polar Net dipole

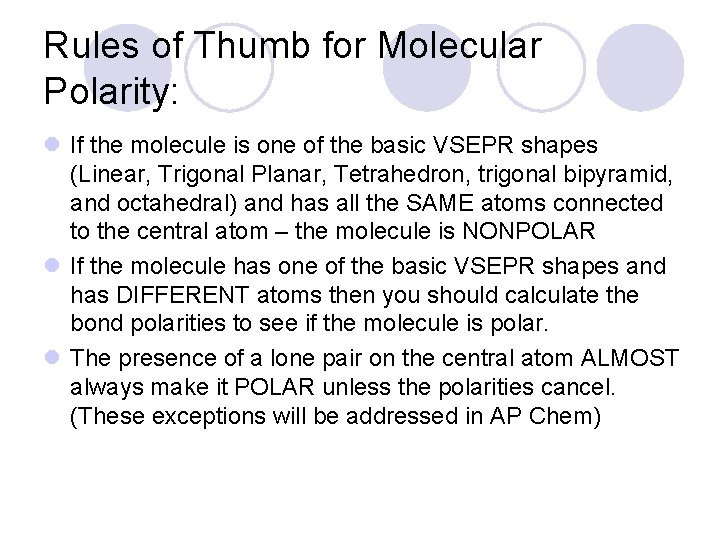
Rules of Thumb for Molecular Polarity: l If the molecule is one of the basic VSEPR shapes (Linear, Trigonal Planar, Tetrahedron, trigonal bipyramid, and octahedral) and has all the SAME atoms connected to the central atom – the molecule is NONPOLAR l If the molecule has one of the basic VSEPR shapes and has DIFFERENT atoms then you should calculate the bond polarities to see if the molecule is polar. l The presence of a lone pair on the central atom ALMOST always make it POLAR unless the polarities cancel. (These exceptions will be addressed in AP Chem)
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Trends of ionization energy
Trends of ionization energy Thinking (electro) negatively answers
Thinking (electro) negatively answers Difference between modern periodic table and mendeleev
Difference between modern periodic table and mendeleev Electronegativity and ph
Electronegativity and ph Polar vs nonpolar
Polar vs nonpolar Atomic radius
Atomic radius The atomic radius in periodic table
The atomic radius in periodic table Least electron affinity
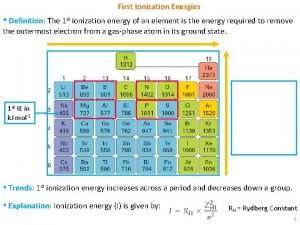
Least electron affinity 1st ionization energy definition
1st ionization energy definition Electronegativity def
Electronegativity def Covalent network vs covalent molecular
Covalent network vs covalent molecular How to determine bond polarity
How to determine bond polarity Electronegativity trend
Electronegativity trend Determining ionization energy
Determining ionization energy Ionization vs electronegativity
Ionization vs electronegativity Electronegativity
Electronegativity Electronegativity
Electronegativity