Electronegativity and Polarity Electronegativity Attracting Electrons When two






- Slides: 29

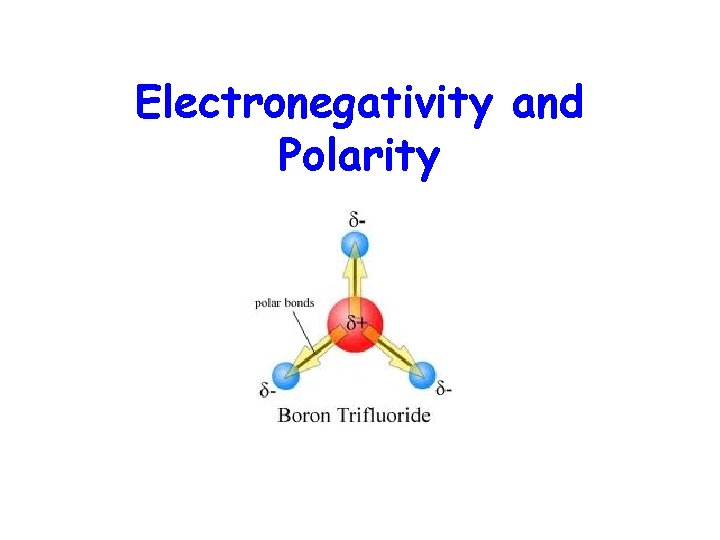
Electronegativity and Polarity

Electronegativity: Attracting Electrons • When two atoms form a bond, each atom attracts the other atom’s electrons in addition to its own. • Electronegativity of an atom is a measure of an atom’s ability to attract electrons in a chemical bond. • As you move from left to right on the periodic table, the EN increases. As you move from top to bottom on the periodic table, the EN decreases.

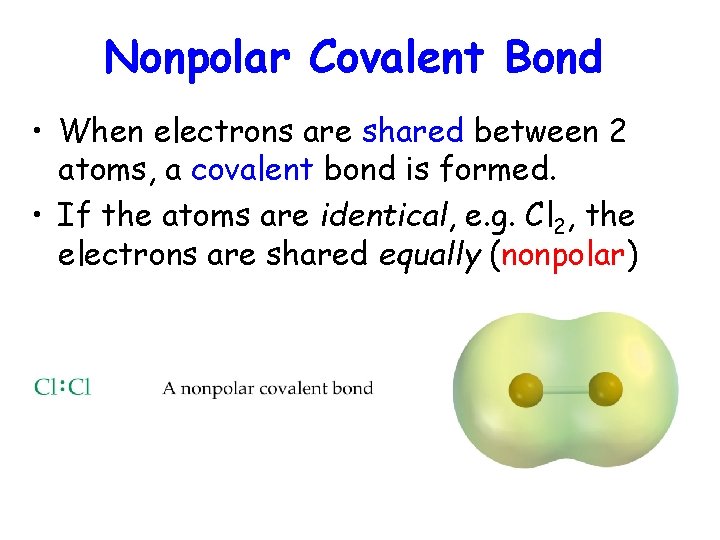
Nonpolar Covalent Bond • When electrons are shared between 2 atoms, a covalent bond is formed. • If the atoms are identical, e. g. Cl 2, the electrons are shared equally (nonpolar)

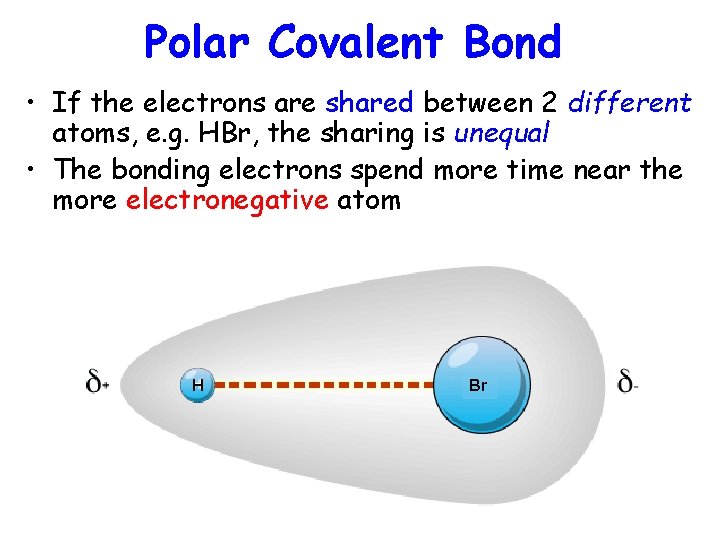
Polar Covalent Bond • If the electrons are shared between 2 different atoms, e. g. HBr, the sharing is unequal • The bonding electrons spend more time near the more electronegative atom H Br

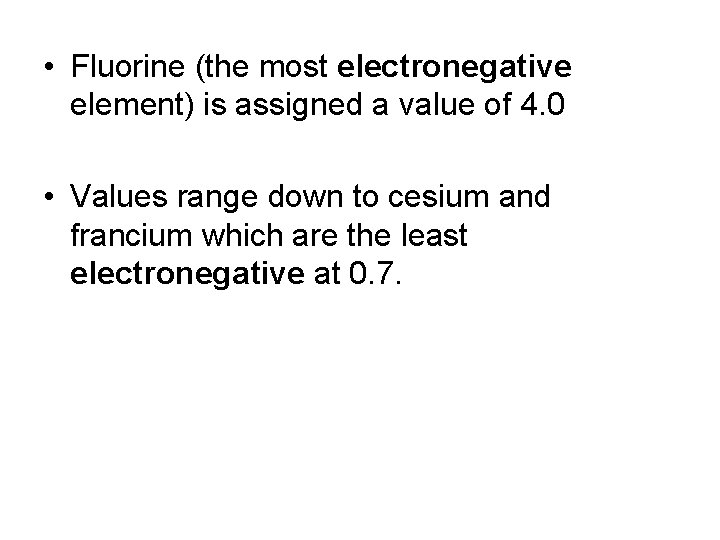
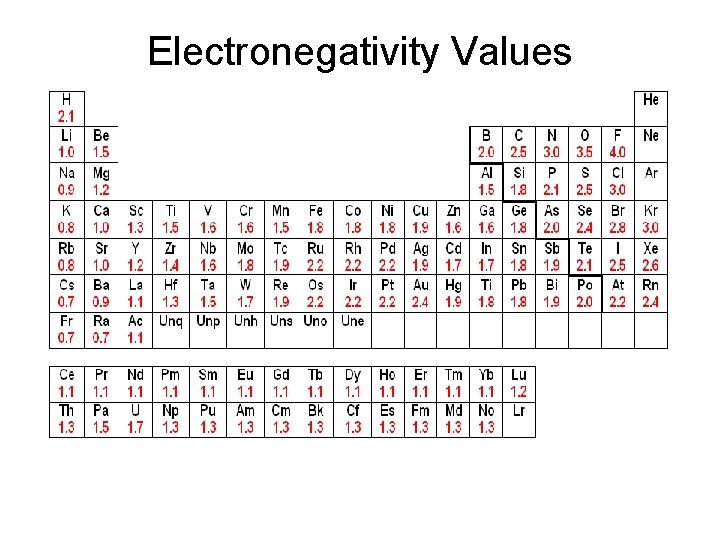
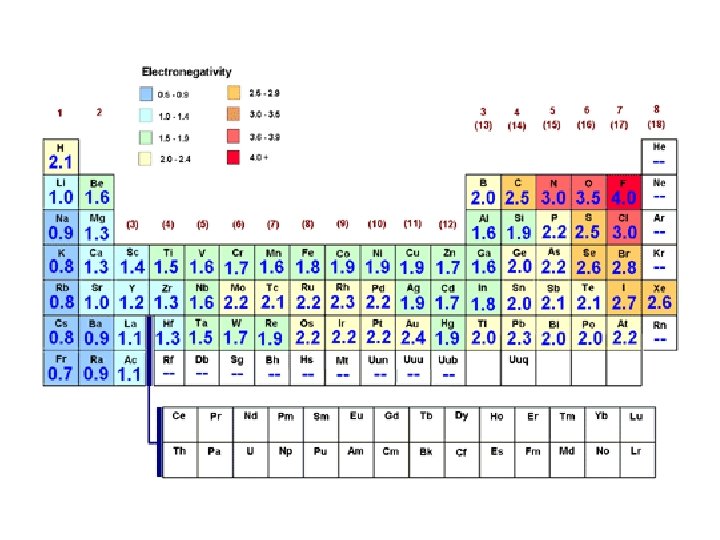
Electronegativity Values • The Pauling scale is the most commonly used – electronegative values assigned to each atom.

• Fluorine (the most electronegative element) is assigned a value of 4. 0 • Values range down to cesium and francium which are the least electronegative at 0. 7.

Electronegativity Values


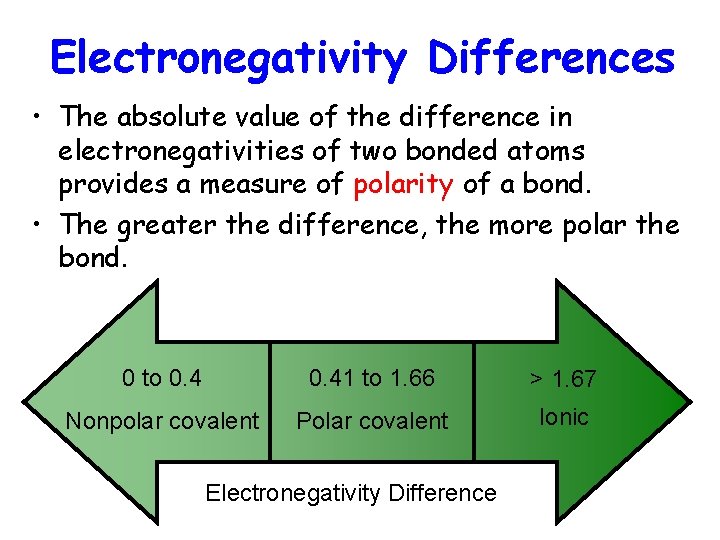
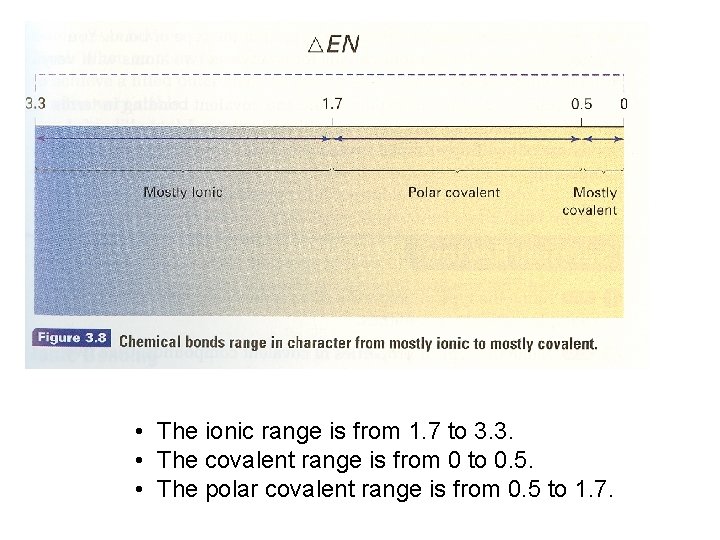
Electronegativity Differences • The absolute value of the difference in electronegativities of two bonded atoms provides a measure of polarity of a bond. • The greater the difference, the more polar the bond. 0 to 0. 41 to 1. 66 > 1. 67 Nonpolar covalent Polar covalent Ionic Electronegativity Difference

• The ionic range is from 1. 7 to 3. 3. • The covalent range is from 0 to 0. 5. • The polar covalent range is from 0. 5 to 1. 7.

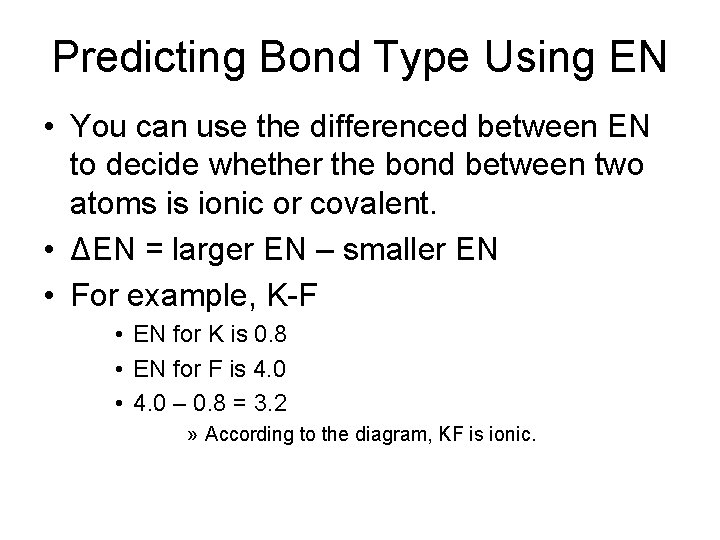
Predicting Bond Type Using EN • You can use the differenced between EN to decide whether the bond between two atoms is ionic or covalent. • ΔEN = larger EN – smaller EN • For example, K-F • EN for K is 0. 8 • EN for F is 4. 0 • 4. 0 – 0. 8 = 3. 2 » According to the diagram, KF is ionic.

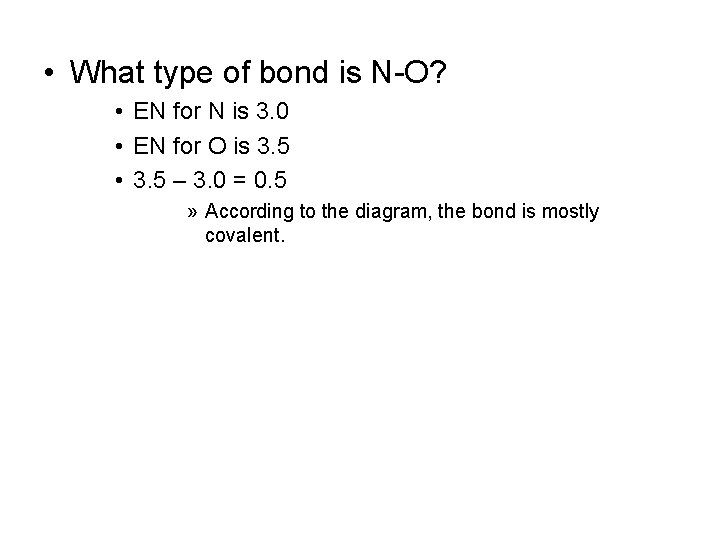
• What type of bond is N-O? • EN for N is 3. 0 • EN for O is 3. 5 • 3. 5 – 3. 0 = 0. 5 » According to the diagram, the bond is mostly covalent.

Polar Covalent Bonds (The in-between bonds) • When two bonding atoms have an EN difference that is greater than 0. 5, but less than 1. 7, they are considered to be polar covalent bonds. • The difference is not great enough for the more EN atom to take the electrons from the less EN atom. • The difference is great enough though, for the bonding electrons to spend more time near the more EN atom.

• Look at the H-O bond in water. • EN for O is 3. 5 • EN for H is 2. 1 • Difference is 1. 4 and this falls in the polar covalent region.

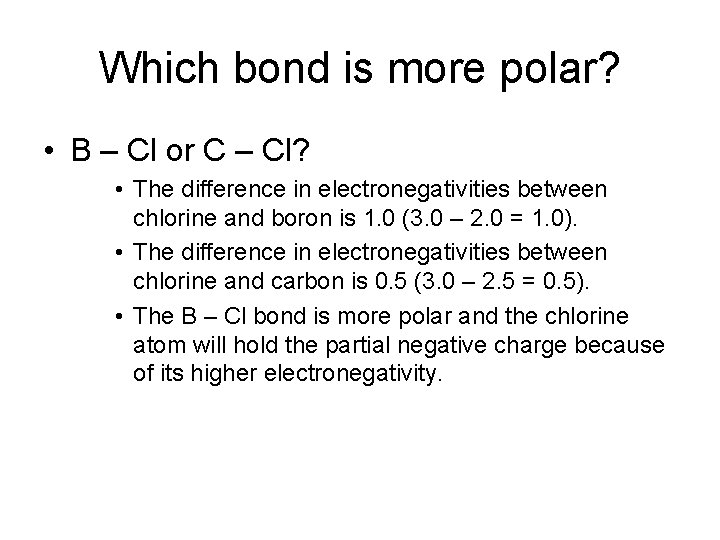
Which bond is more polar? • B – Cl or C – Cl? • The difference in electronegativities between chlorine and boron is 1. 0 (3. 0 – 2. 0 = 1. 0). • The difference in electronegativities between chlorine and carbon is 0. 5 (3. 0 – 2. 5 = 0. 5). • The B – Cl bond is more polar and the chlorine atom will hold the partial negative charge because of its higher electronegativity.

Which bond is more polar? • P – F or P – Cl? • The difference in electronegativities between fluorine and phosphorus is 1. 9 (4. 0 – 2. 1 = 1. 9). • The difference in electronegativities between chlorine and phosphorus is 0. 9 (3. 0 – 2. 1 = 0. 9). • The P – F bond is more polar and the fluorine atom will hold the partial negative charge because of its higher electronegativity.

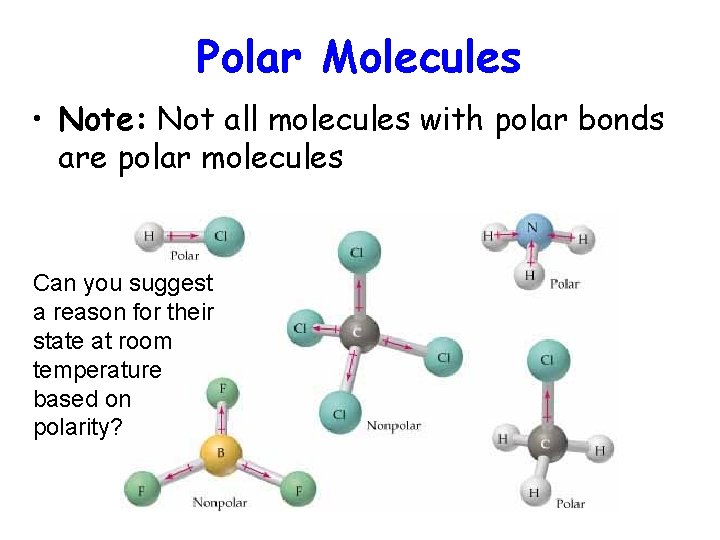
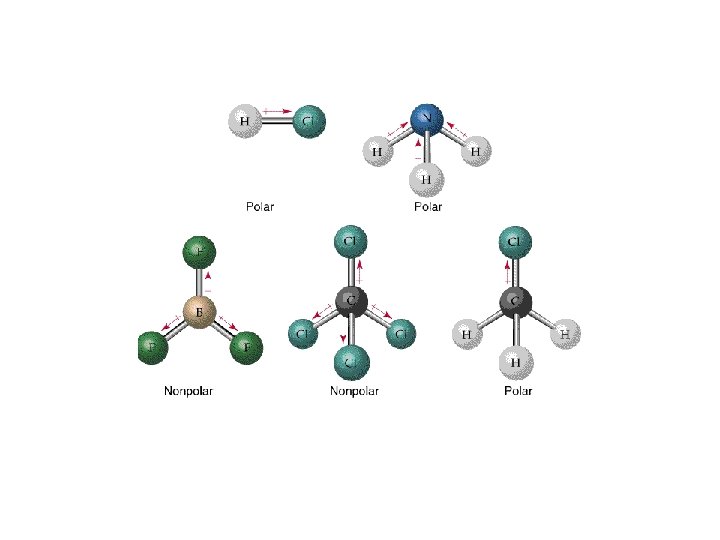
Polar Molecules • Note: Not all molecules with polar bonds are polar molecules Can you suggest a reason for their state at room temperature based on polarity?

Metallic Bonding • When metals combine with other metals, they do not have an EN greater than 1. 7, does that mean that they are covalent? • Sodium metal is soft enough to be cut with a butter knife, copper and gold can be drawn into thin wires and hammered into sheets (ionic bonds are brittle), does this mean they have covalent bonds? • No. Metals do not have enough valance electrons to achieve stable configurations by sharing electrons.

• The force that holds metal atoms together is called a metallic bond. • Unlike covalent and ionic bonding, metallic bonding does not have a particular orientation in space. Electrons are free to move and the metal ions are not rigidly held in formation. This is why a hammer can pound metal – the atoms can slide past each other.


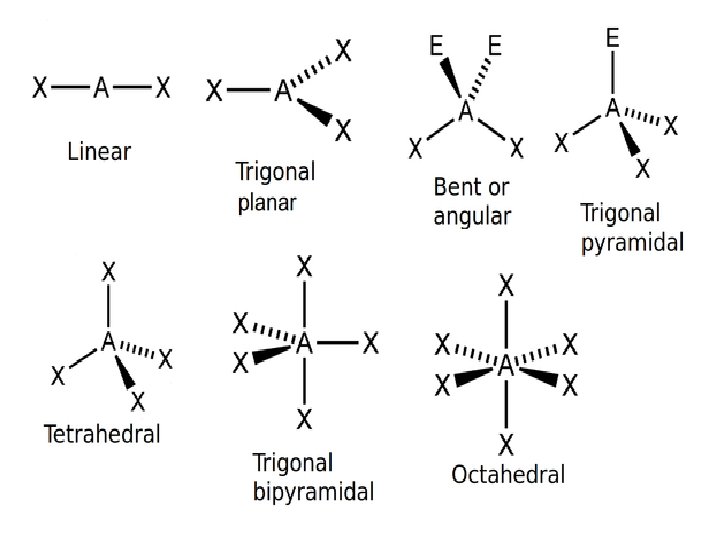
Molecular Shapes and Molecular Polarity • Bond polarity is a measure of how equally the electrons in a bond are shared between two atoms. • As the difference in electronegativity between two atoms increases, so does bond polarity.

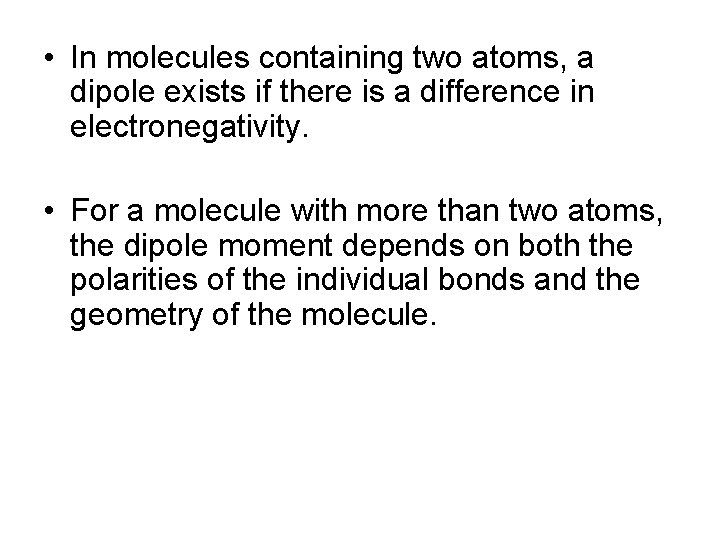
• In molecules containing two atoms, a dipole exists if there is a difference in electronegativity. • For a molecule with more than two atoms, the dipole moment depends on both the polarities of the individual bonds and the geometry of the molecule.

• Consider linear CO 2 • Each C=O bond is polar, but since the bonds are identical, the bond dipoles are equal in magnitude. The overall dipole moment = 0.

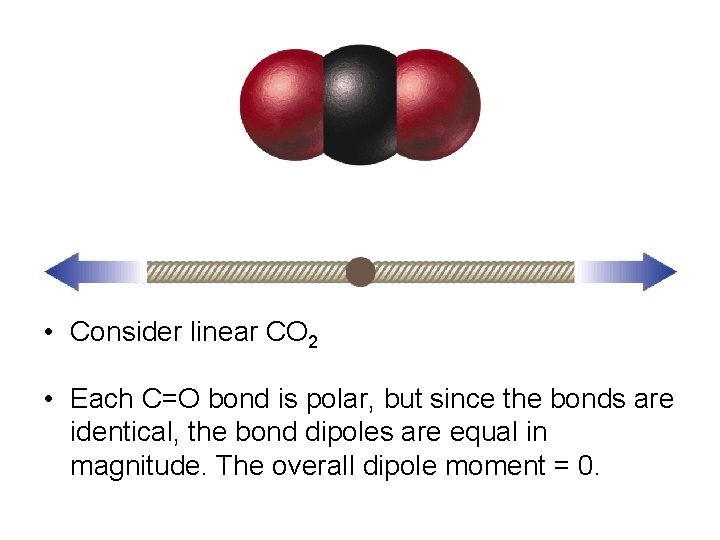
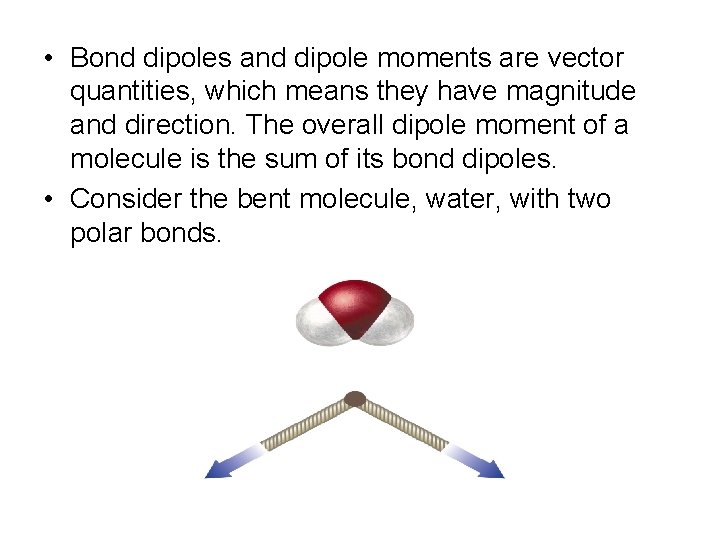
• Bond dipoles and dipole moments are vector quantities, which means they have magnitude and direction. The overall dipole moment of a molecule is the sum of its bond dipoles. • Consider the bent molecule, water, with two polar bonds.

• Both bonds are identical so the bond dipoles are equal, but since the molecule is bent, the bonds do not directly oppose each other. Therefore, the bond dipoles do not cancel each other out. • The water molecule has a non zero dipole moment so it is polar. The oxygen carries a partial negative charge and the hydrogen atoms each carry partial positive charges.


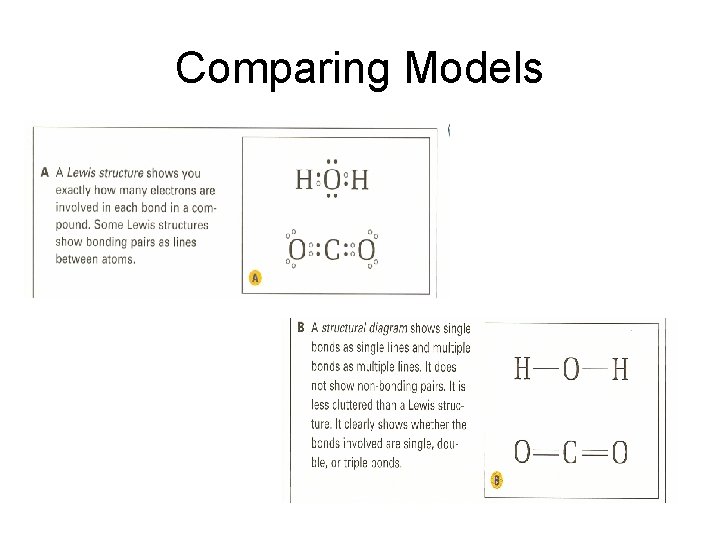
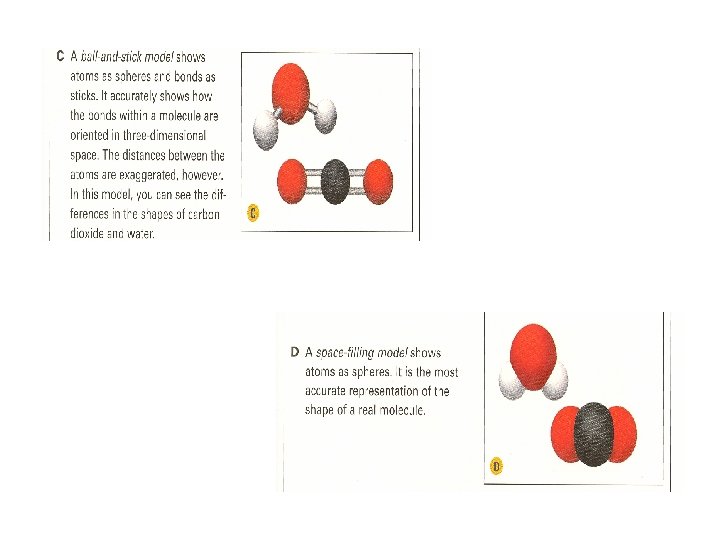
Comparing Models

