Properties of Liquids Hold your cursor over the































- Slides: 45

Properties of Liquids

Hold your cursor over the image to see molecular state of the underlying material.




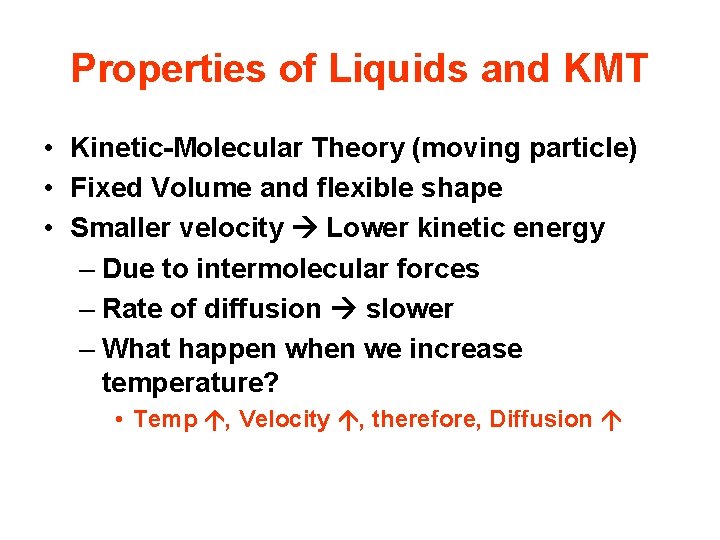
Properties of Liquids and KMT • Kinetic-Molecular Theory (moving particle) • Fixed Volume and flexible shape • Smaller velocity Lower kinetic energy – Due to intermolecular forces – Rate of diffusion slower – What happen when we increase temperature? • Temp , Velocity , therefore, Diffusion

Properties of Liquids • Particles are much closer together – Why: Attractive forces are more effective – How: Dipole-dipole, London dispersion (instant dipole) and hydrogen bonding – So? Relatively high density (more mass in a specific volume) compared to gas – Denser than solid (exception: Water) – And? Relative incompressibility

Properties of Liquids • More ordered than gases due to strong intermolecular forces • Particles are not bound in fixed positions – Constantly moving Fluid (ability to flow) • Most liquid flow downhill due to gravity • Few do flow uphill (unusual) – Example: liquid Helium near absolute zero

Intermolecular Forces and the Properties of Liquids In summary, intermolecular forces play a large role in many of the physical properties of liquids and gases. These include: • • • vapor pressure boiling point surface tension capillary action viscosity

Intermolecular Forces and the vapor pressure • The vapor pressure of a liquid depends on intermolecular forces. • When the intermolecular forces in a liquid are strong, you expect the vapor pressure to be low.

Intermolecular Forces and the normal boiling point • The normal boiling point is related to vapor pressure and is lowest for liquids with the weakest intermolecular forces. • When intermolecular forces are weak, little energy is required to overcome them. • Consequently, boiling points are low for such compounds.

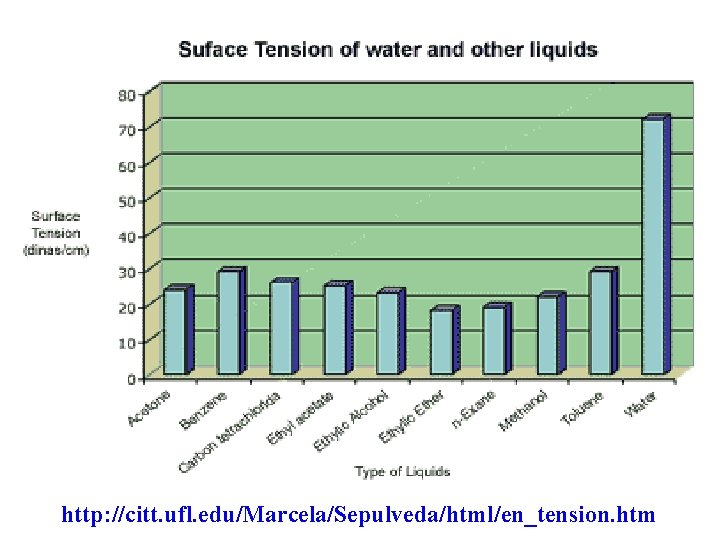
Intermolecular Forces and the surface tension • Surface tension increases with increasing intermolecular forces. • Surface tension is the energy needed to increase the surface area of a liquid. • To increase surface area, it is necessary to pull molecules apart against the intermolecular forces of attraction.

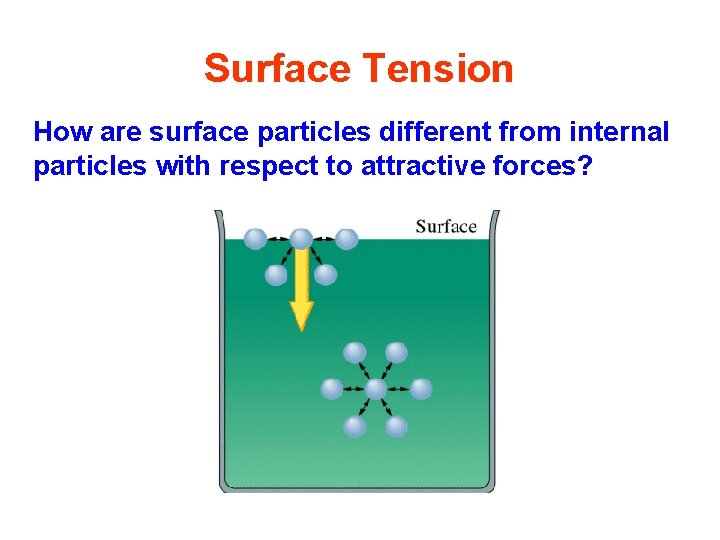
Surface Tension How are surface particles different from internal particles with respect to attractive forces?

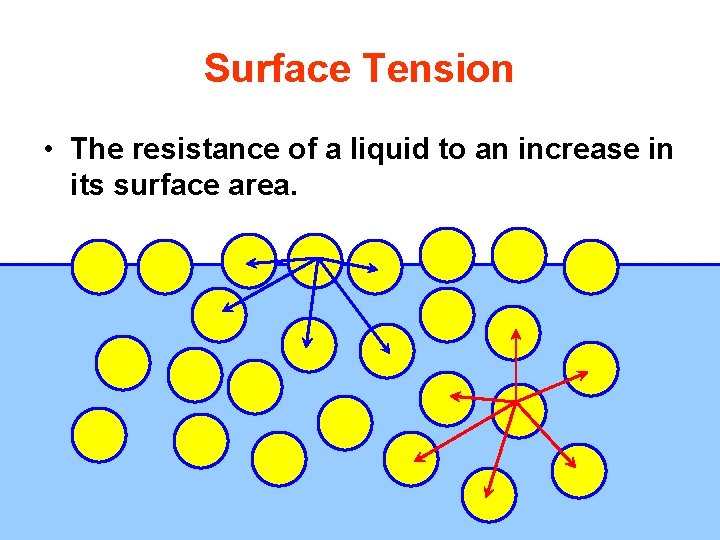
Surface Tension • The resistance of a liquid to an increase in its surface area.

Surface Tension 1. Attraction of surface 2. molecules that cause the liquid 3. surface to contract and become 4. more spherical 5. 2. Amount of energy required to 6. stretch or increase the surface

Surface Tension – Common to ALL liquids – A force tends to pull adjacent parts of a liquid’s surface together – Directly related to the force of attraction between particles – Decreasing surface area to the smallest possible size – Liquid droplets are spherical • Reason: Least surface area

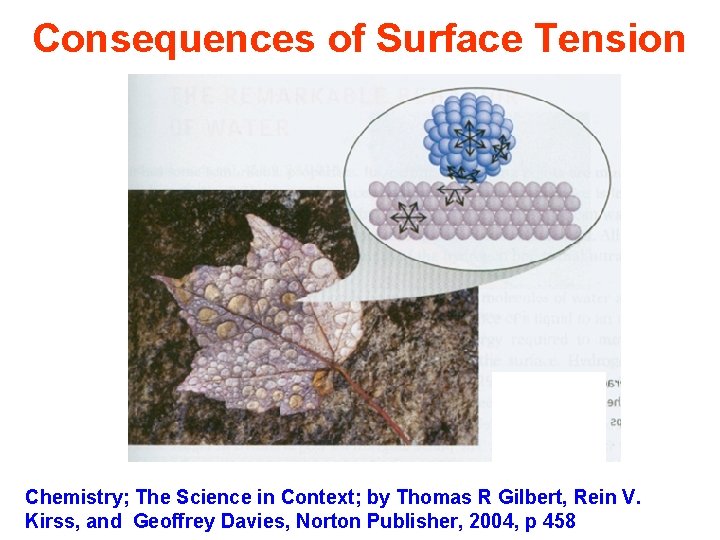
Consequences of Surface Tension Chemistry; The Science in Context; by Thomas R Gilbert, Rein V. Kirss, and Geoffrey Davies, Norton Publisher, 2004, p 457

Consequences of Surface Tension Chemistry; The Science in Context; by Thomas R Gilbert, Rein V. Kirss, and Geoffrey Davies, Norton Publisher, 2004, p 458

http: //citt. ufl. edu/Marcela/Sepulveda/html/en_tension. htm



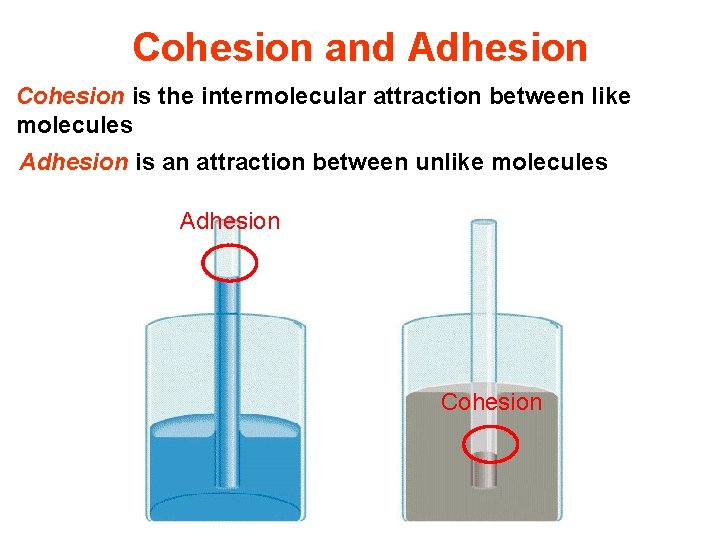
Capillary action • To understand how capillary action works, we must first differentiate between cohesion and adhesion. – Cohesive forsce: intermolecular forces that bind similar molecules to one another, such as the hydrogen bonding in water, are called cohesive forces. – Adhesive forces: intermolecular forces that bind a substance to a surface are called adhesive forces.

Cohesion and Adhesion Cohesion is the intermolecular attraction between like molecules Adhesion is an attraction between unlike molecules Adhesion Cohesion

Cohesion/Adhesion • Why is this so? • In mercury the cohesive forces are stronger than the adhesive forces to glass.

Cohesion/Adhesion • Water tends to “wet” glass because the adhesive forces between the liquid molecules and the glass molecules are greater than the cohesive forces between the liquid molecules. • Water beads up on a wax surface because the cohesive forces between the water molecules are greater than the adhesive forces between the water molecules and the wax molecules.

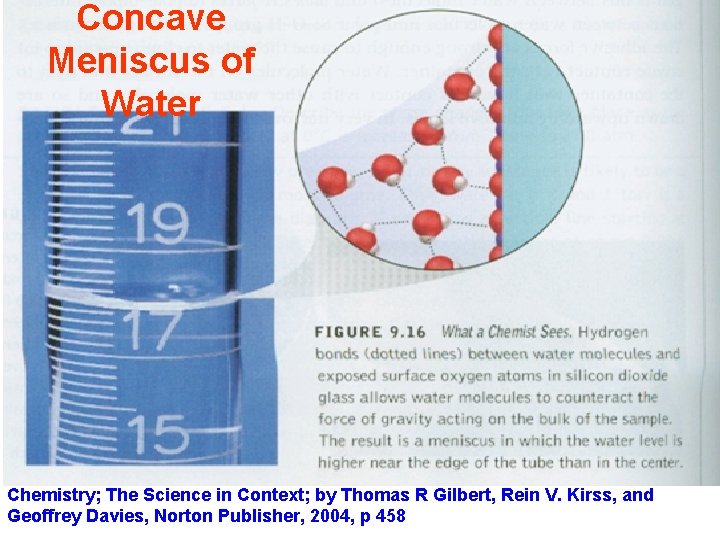
Concave Meniscus of Water Chemistry; The Science in Context; by Thomas R Gilbert, Rein V. Kirss, and Geoffrey Davies, Norton Publisher, 2004, p 458

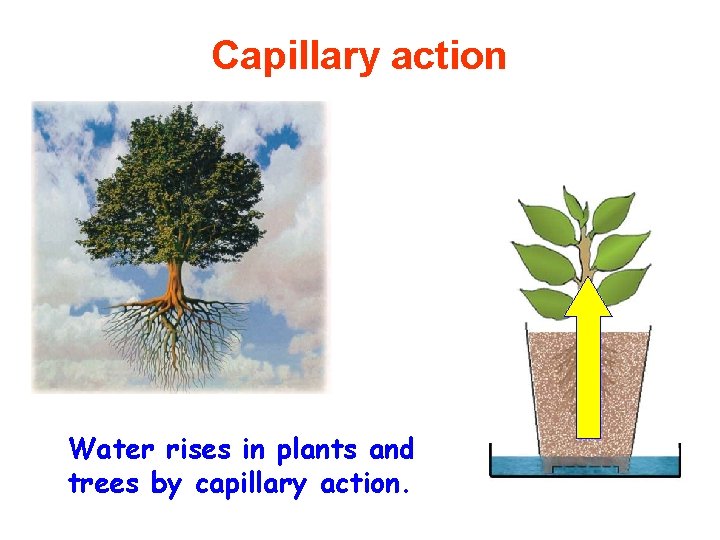
Capillary action – Attraction of the surface of a liquid to the surface of a solid – Pull liquid molecules upward along the surface against the pull of gravity – Why so important? • Transportation of water from roots of a plant to its leaves in the opposite direction to gravity

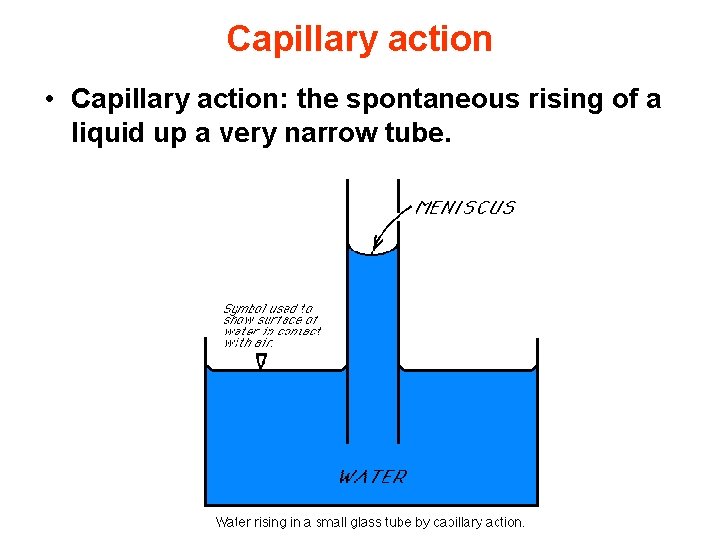
Capillary action • Capillary action: the spontaneous rising of a liquid up a very narrow tube.

Capillary action ; Rise of Liquid up a Very Small Diameter Glass Tube Chemistry; The Science in Context; by Thomas R Gilbert, Rein V. Kirss, and Geoffrey Davies, Norton Publisher, 2004, p 459

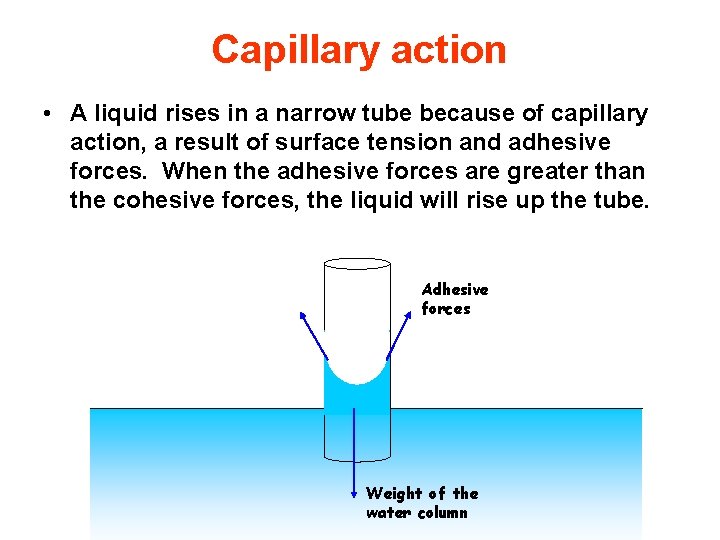
Capillary action • A liquid rises in a narrow tube because of capillary action, a result of surface tension and adhesive forces. When the adhesive forces are greater than the cohesive forces, the liquid will rise up the tube. Adhesive forces Weight of the water column

Capillary action • When cohesive forces between molecules of the liquid exceed adhesive forces, the liquid level in the capillary tube is below the surface of the surrounding fluid.

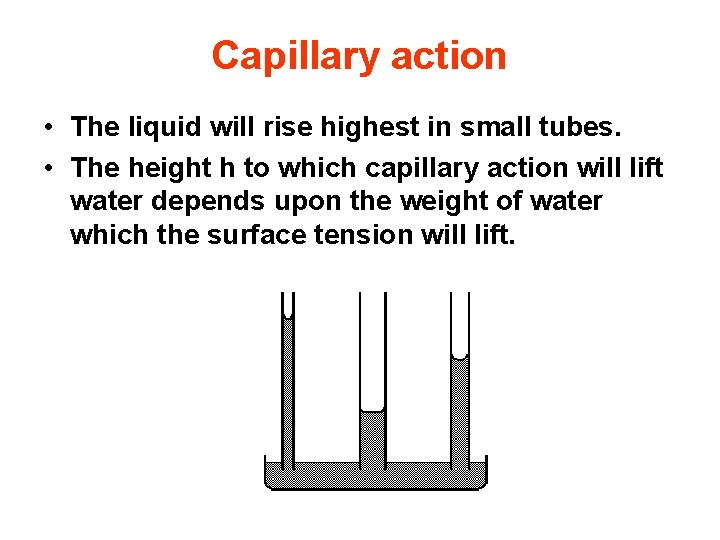
Capillary action • The liquid will rise highest in small tubes. • The height h to which capillary action will lift water depends upon the weight of water which the surface tension will lift.

Capillary action • When these forces balance the water will stop rising.

Capillary action Hot wax is pulled up the wick by capillary action. It is the wax that burns, not the wick.

Capillary action Water rises in plants and trees by capillary action.


Viscosity • The resistance of a liquid to flow is called its viscosity. • The greater a liquid’s viscosity, the more slowly it flows. • Viscosity is related to the ease with which individual molecules of the liquid can move with respect to one another. • It depends on the attractive forces between molecules and on whether structural features exist that cause the molecules to become entangled.

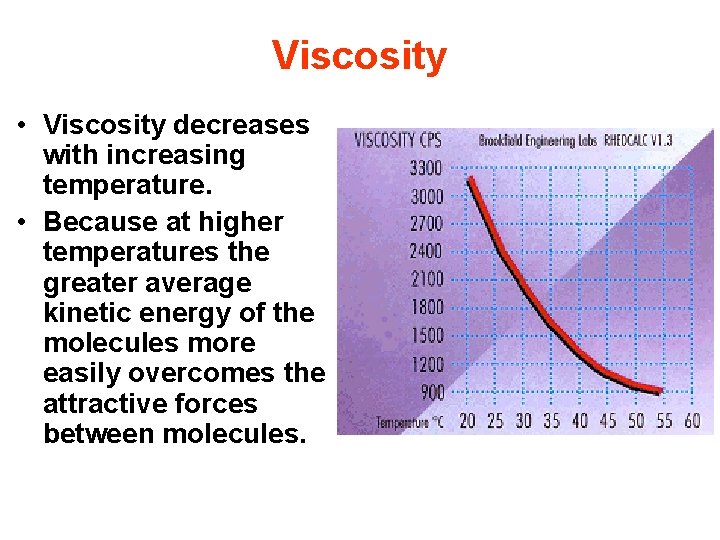
Viscosity • Viscosity decreases with increasing temperature. • Because at higher temperatures the greater average kinetic energy of the molecules more easily overcomes the attractive forces between molecules.

Viscosity – Resistance to Flow – Increase Intermolecular Force; Increase Viscosity – Increase Temperature; Decrease Intermolecular Force; Decrease Viscosity

Intermolecular Forces and the viscosity • Viscosity increases with increasing intermolecular forces because increasing these forces increases the resistance to flow. • Other factors, such as the possibility of molecules tangling together, affect viscosity. • Liquids with long molecules that tangle together are expected to have high viscosities.

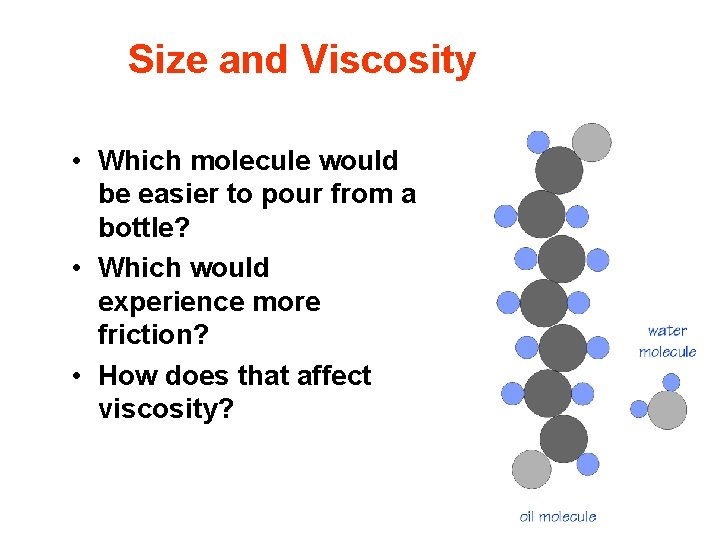
Size and Viscosity • Which molecule would be easier to pour from a bottle? • Which would experience more friction? • How does that affect viscosity?

Size and Viscosity • When looking at the molecules on the slide it is easy to see that the oil molecule is much more likely to become entangled with other oil molecules when being poured. This is vital to viscosity. • Consider the shape of these two molecules. The oil molecule is quite long and somewhat thick. The water molecule in comparison is compact and small. Will they actually pour? Experience tells us yes. • Being poured is pretty much like rolling downhill. The molecules bump into one another as they tumble out of the bottle.

Size and Viscosity • If we think of the oil as being shaped close to a big thick tree and the water like a almost round boulder, which one would roll downhill easier? The boulder. Why? • The big heavy tree would experience more friction. Now imagine billions of big oil molecules trying to roll down at the same time – Log Jam, lots of friction and the process would take a while. In other words, it would resist flow. • A bunch of roundish boulders on the other hand would make for a pretty easy roll downhill and in fact landslides and avalanches are a real problem in some parts of the world. The boulders would resist the flow much less than logs with big branches on them.

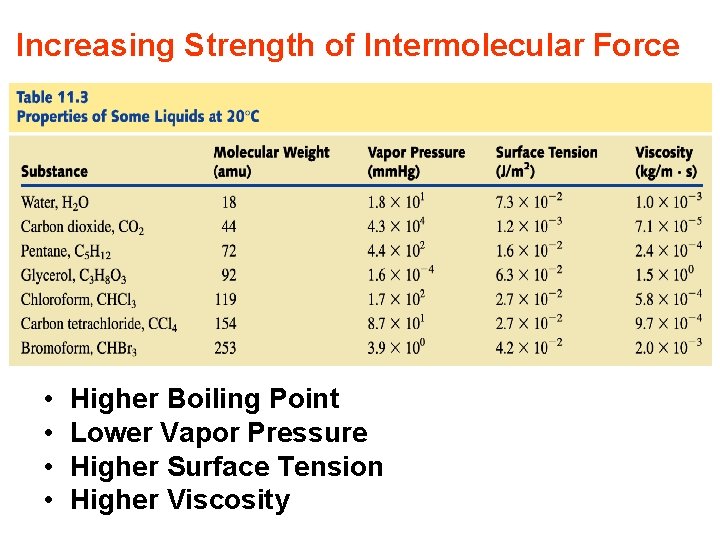
Increasing Strength of Intermolecular Force • • Higher Boiling Point Lower Vapor Pressure Higher Surface Tension Higher Viscosity

REFERENCES • http: //www. media. pearson. com. au/schools/cw/au_sc h_whalley_sf 1_1/int/matter. html • http: //www. footprints-science. co. uk/states. htm • http: //www. chem. purdue. edu/gchelp/atoms/states. ht ml
 Do
Do Buoyancyability
Buoyancyability Properties of solid
Properties of solid Properties of a solid
Properties of a solid Matter and its composition
Matter and its composition Cursor motion blur windows
Cursor motion blur windows The cursor movie
The cursor movie Pl sql cursor
Pl sql cursor Excel white cross cursor
Excel white cross cursor Cursor fast forward sql server
Cursor fast forward sql server 2 147 483
2 147 483 Reostat značka
Reostat značka L cursor
L cursor Tweepy.cursor(api.search
Tweepy.cursor(api.search Where current of cursor
Where current of cursor Will your anchor hold
Will your anchor hold Sing together lyrics
Sing together lyrics Hold your horses idiom meaning and sentence
Hold your horses idiom meaning and sentence Holding onto faith
Holding onto faith Hold fast to your dreams for without them life is a broken
Hold fast to your dreams for without them life is a broken Rise up like an eagle
Rise up like an eagle Hide me now under your wing
Hide me now under your wing Hold on to your future
Hold on to your future I want to hold your hand analysis
I want to hold your hand analysis Lord i come to you
Lord i come to you Over the mountain over the plains
Over the mountain over the plains Siach reciting the word over and over
Siach reciting the word over and over Handing over and taking over the watch
Handing over and taking over the watch Give us your hungry your tired your poor
Give us your hungry your tired your poor Extensive vs intensive
Extensive vs intensive Is smell a physical property
Is smell a physical property Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan