Liquids and Solids Properties of Liquids and the



















- Slides: 35

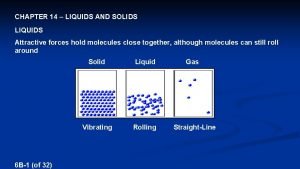
Liquids and Solids

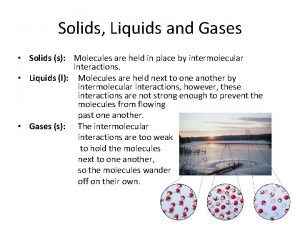
Properties of Liquids and the Kinetic. Molecular Theory • Liquid- is a form of matter that has a definite volume and takes the shape of its container. The particles are in constant motion. • The intermolecular forces in liquids can be dipole, London dispersion, and hydrogen bonding. • The Kinetic-Molecular Theory states that particles of a liquid have no fixed space, and move about constantly. • Fluid-is a substance that can flow and takes the shape of its container- used for liquids and gases both (Showing meting of ice and changes in molecular structure) http: //mutuslab. cs. uwindsor. ca/schurko/animations/waterphases/status_water. htm

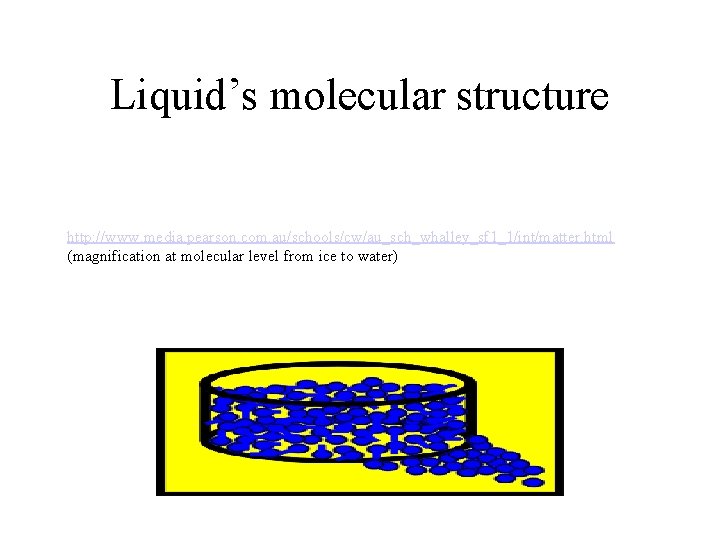
Liquid’s molecular structure http: //www. media. pearson. com. au/schools/cw/au_sch_whalley_sf 1_1/int/matter. html (magnification at molecular level from ice to water)

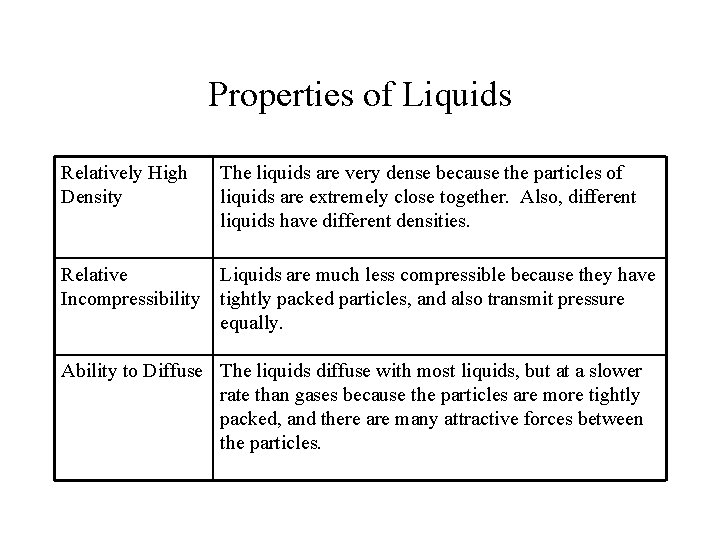
Properties of Liquids Relatively High Density The liquids are very dense because the particles of liquids are extremely close together. Also, different liquids have different densities. Relative Liquids are much less compressible because they have Incompressibility tightly packed particles, and also transmit pressure equally. Ability to Diffuse The liquids diffuse with most liquids, but at a slower rate than gases because the particles are more tightly packed, and there are many attractive forces between the particles.

Intermolecular Forces: Van Der Waals Forces • Significant in molecular substances (gases, most liquids and solids that are molecular) • Strong intermolecular forces lead to increased m. p. and b. p. • What are some properties of molecular substances? (nonconductors, insoluble in water, but soluble in most non polar solvents) http: //www. wwnorton. com/COLLEGE/chemistry/gilbert/tutorials/interface. asp? ch apter=chapter_09&folder=intermolecular_forces (ion-ion, ion- dipole, dipole-dipole tutorial)

LDFs -temporary dipole interactions and are the weakest intermolecular bonds -present between all molecules and non bonded atoms, and are significant in noble gas atoms and non polar compounds. -The strength of LDFs depends upon two things: 1. # of e in atoms that make up the molecule 2. The ease with which e are dispersed to form temporary dipoles. http: //antoine. frostburg. edu/chem/senese/101/liquids/faq/h-bonding-vs-london-forces. shtml Dipole-Dipole, LDFs animation

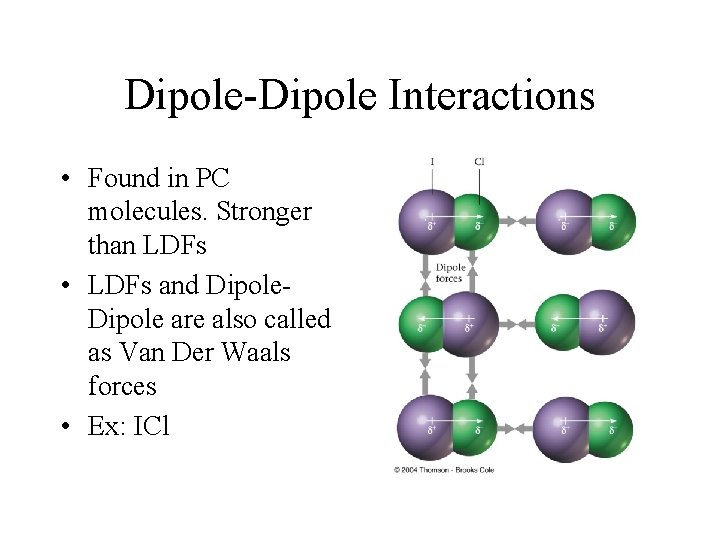
Dipole-Dipole Interactions • Found in PC molecules. Stronger than LDFs • LDFs and Dipole are also called as Van Der Waals forces • Ex: ICl

H Bonds • When H is bonded to a relatively small, electronegative atom, such as N, O, or F • Strongest of the weak interactions (strongest intermolecular forces)

Properties of Liquids: Surface Tension http: //www. visionlearning. com/library/module_viewer. php? mid=57 Water Strider Video

Surface Tension and Capillary Action http: //www. wwnorton. com/COLLEGE/chemistry/gilbert/tutorials/interface. asp? chapter=chapter_09&folde r=capillary_action (Capillary, Surface tension Tutorial) Surface Tension-a force that tends to pull adjacent parts of a liquid’s surface together, thereby decreasing surface area to the smallest possible size. ~The higher the attraction forces (intermolecular forces), the higher the surface tension. Surface tension causes liquid droplets to take a spherical shape. Capillary action- the attraction of the surface of a liquid to the surface of a solid. ~Capillary action is the reason water from the roots of a tree goes to the leaves. It is also responsible for the liquid surface called the meniscus.

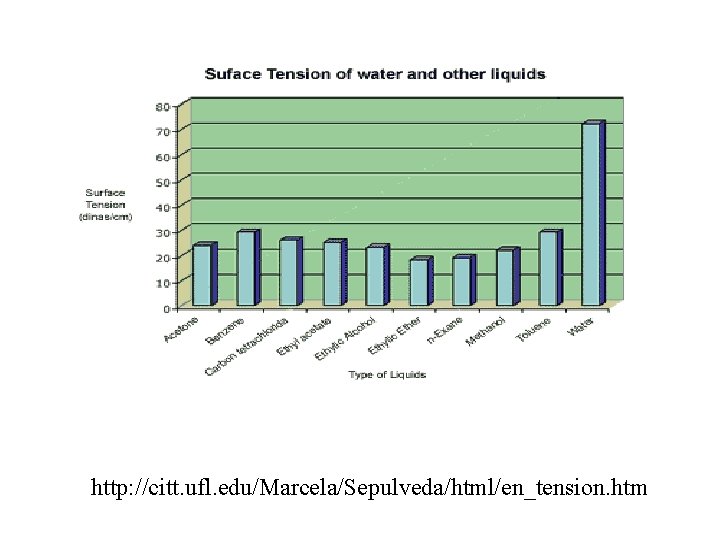
Surface Tension • The surface of any liquid behaves as if it was a stretched membrane. This phenomenon is known as surface tension • Surface tension is caused by intermolecular forces at the liquid’s interface with a gas or a solid. • Surface tension depends on the nature of the liquid, the surrounding media and temperature. • Liquids that have strong intermolecular forces will have higher values of surface tension than liquids that have weak intermolecular forces.


http: //citt. ufl. edu/Marcela/Sepulveda/html/en_tension. htm

Viscosity • Defined as “resistance to flow” of a fluid. • Viscous liquids move slower. • The greater the intermolecular forces the more is the viscosity. http: //plc. cwru. edu/tutorial/enhanced/lab/visco/intro. htm (Viscosity and molecular size interactive simulation) http: //www. youtube. com/watch? v=7 Ft 9 VDDPWb 4&feature=related (video on different density liquids)

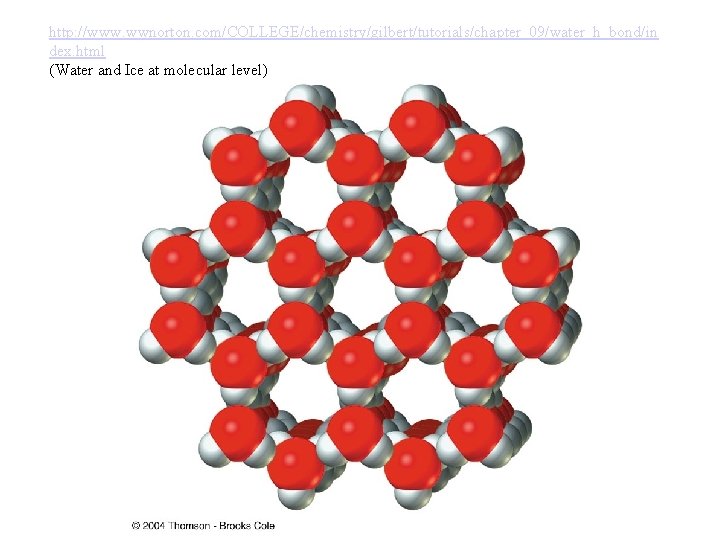
Physical Properties of Water • Highest density at 4 degrees celsius. Ice is one of the few solids to have a lower density in solid phase as compared to liquid phase. This property is very useful in ice skating, and fishes in lakes etc. • Because of H Bonding water has a much higher b. p. and m. p. as compared to other liquids. This property is making water one of the best coolants. • ( Ex: Perspiration, In car radiators etc)

http: //www. wwnorton. com/COLLEGE/chemistry/gilbert/tutorials/chapter_09/water_h_bond/in dex. html (Water and Ice at molecular level)

Liquid- Vapor Equilibrium • Vapor Pressure: The pressure exerted on the surface of a liquid by the vapor that is in equilibrium with the liquid is called as “vapor pressure” • Once equilibrium between a liquid and vapor is reached, the number of molecules per unit volume in a vapor does not change with time. Hence, the vapor pressure over the liquid remains constant at a given temperature. • Vapor Pressure is independent of the volume of the container. Why? http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flash/vaporv 3. swf (vapor pressure equilibrium on manometer)

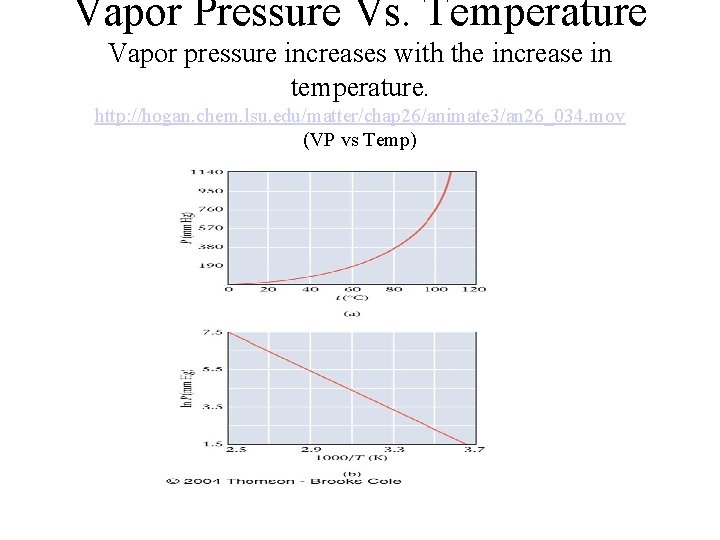
Vapor Pressure Vs. Temperature Vapor pressure increases with the increase in temperature. http: //hogan. chem. lsu. edu/matter/chap 26/animate 3/an 26_034. mov (VP vs Temp)

Evaporation Vaporization-the process by which a liquid or solid changes to gas. Evaporation- is the process where particles escape from the surface of a non boiling liquid and enters the gas state. ~Evaporation takes place because the particles of liquids have different kinetic energies, therefore some of the particles with higher kinetic energy overcome the intermolecular forces and evaporate to go in the gas phase.

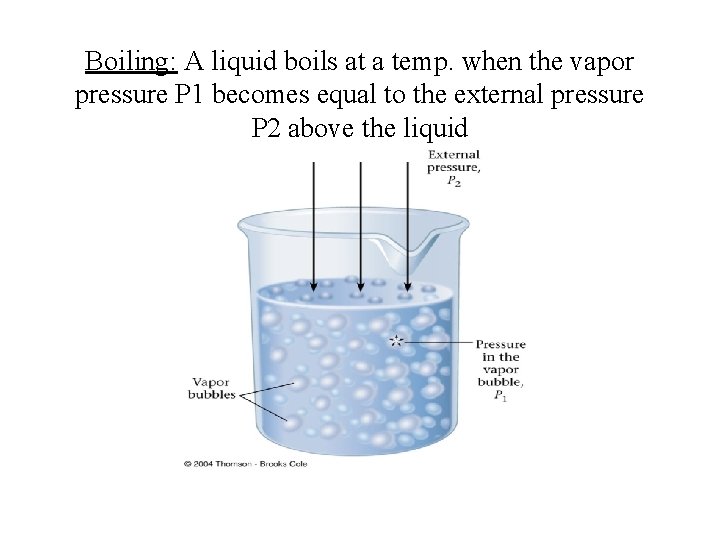
Boiling- is the change of a liquid to bubbles or vapor. Boiling occurs when the vapor pressure becomes equals atmospheric pressure. • A liquid boils at the temp. at which its vapor pressure is equal to the pressure above its surface. (usually atmospheric pressure) • If the pressure above the liquid’s surface is 1 atm, then this temperature is called as its “Normal Boiling Point” • B. P. of a liquid is reduced by lowering the pressure above it. • Why does it take longer to cook at high altitudes?

Boiling: A liquid boils at a temp. when the vapor pressure P 1 becomes equal to the external pressure P 2 above the liquid

Phase Changes • Melting(fusion)/Freezing • Vaporization/Condensation • Sublimation/Deposition http: //hogan. chem. lsu. edu/matter/chap 26/animate 3/an 26_035. mov (s-l-g with molecular motion at phase change)

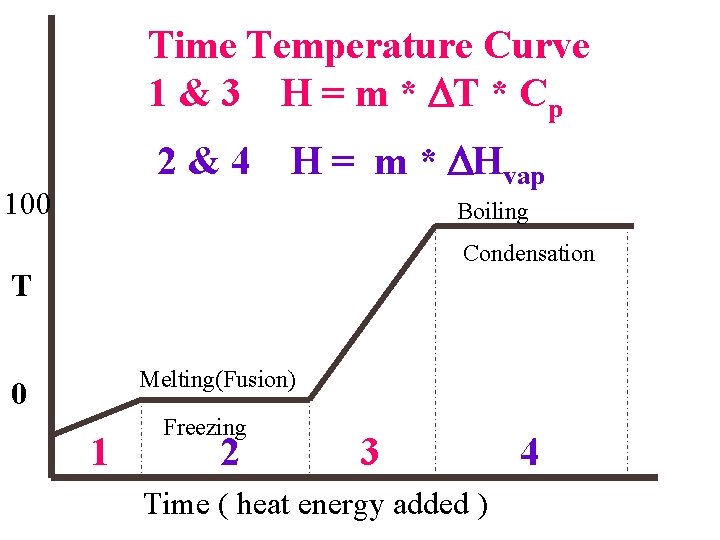
Time Temperature Curve 1 & 3 H = m * DT * Cp 2&4 100 H = m * DHvap Boiling Condensation T Melting(Fusion) 0 1 Freezing 2 3 Time ( heat energy added ) 4

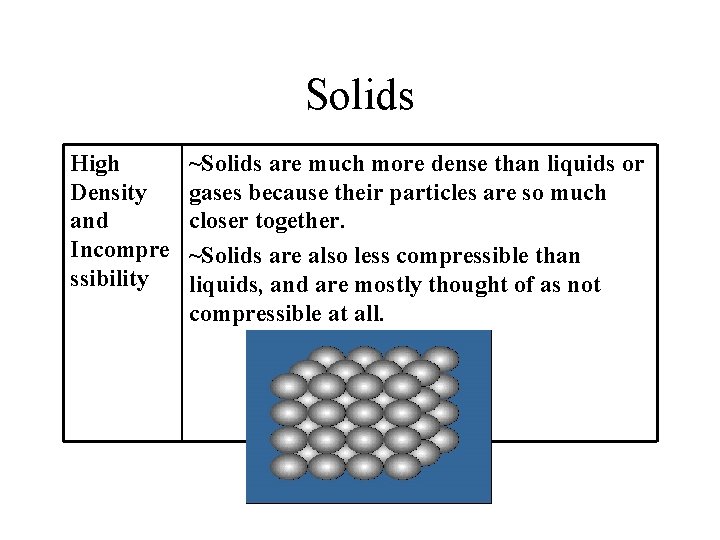
Solids High Density and Incompre ssibility ~Solids are much more dense than liquids or gases because their particles are so much closer together. ~Solids are also less compressible than liquids, and are mostly thought of as not compressible at all.

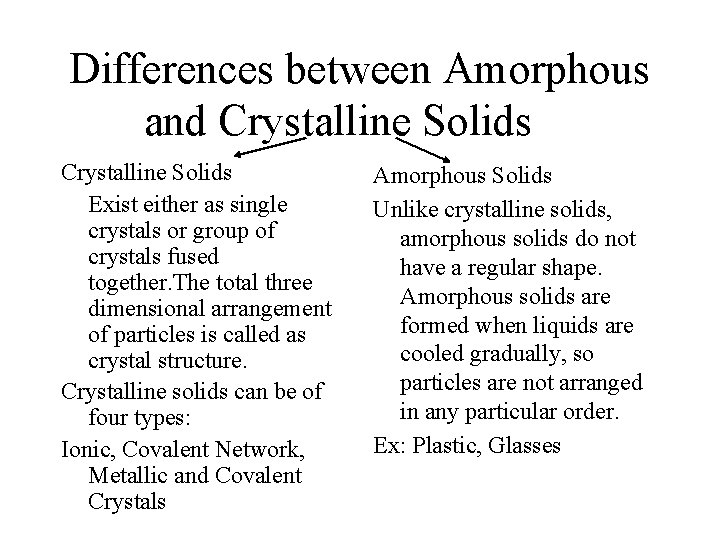
Types of Solids • Solids are of two types: • Crystalline solids- consist of crystals which are substances which are organized in symmetric, geometric ways. • Amorphous solids-non crystalline solid where the particles are arranged randomly.

Differences between Amorphous and Crystalline Solids Exist either as single crystals or group of crystals fused together. The total three dimensional arrangement of particles is called as crystal structure. Crystalline solids can be of four types: Ionic, Covalent Network, Metallic and Covalent Crystals Amorphous Solids Unlike crystalline solids, amorphous solids do not have a regular shape. Amorphous solids are formed when liquids are cooled gradually, so particles are not arranged in any particular order. Ex: Plastic, Glasses

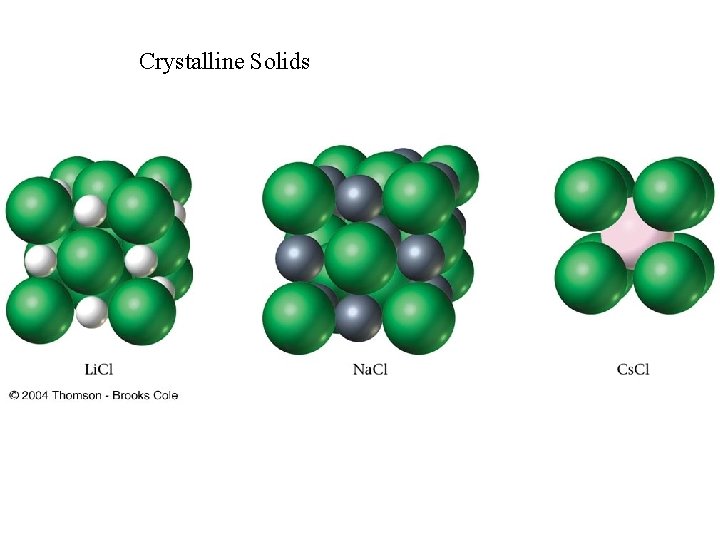
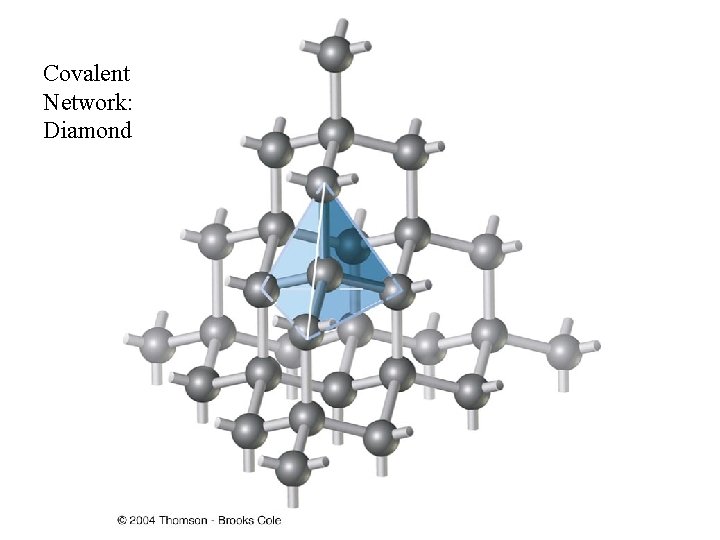
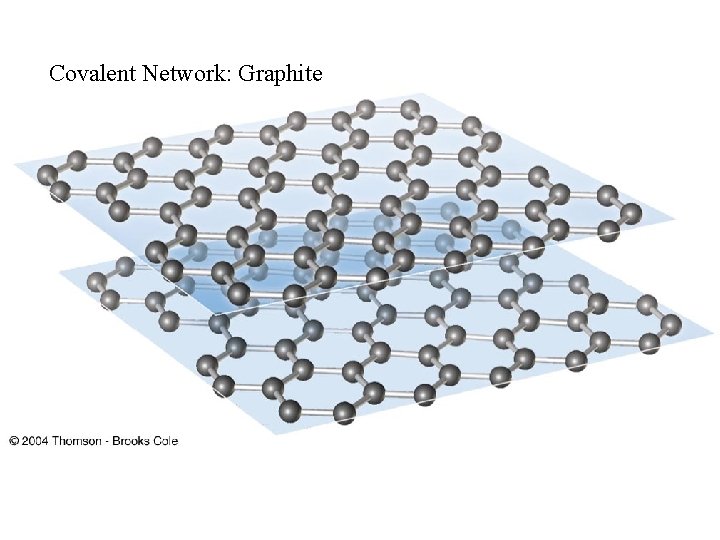
Crystalline Solids • There are four types of crystals: • 1. Ionic crystals: The positive and negative ions crystals have properties: high melting points, are hard and brittle, and good insulators. • 2. Covalent network crystals: the sites have single atoms. They are nonconductors or semiconductors and have high melting points. Ex: Graphite, diamond • 3. Metallic crystals: these are metal atoms with a sea of valence electrons. There is high electric conductivity of metals, and the melting points differ. Ex: Iron, Aluminum etc • 4. Covalent molecular crystals: they are held together by covalent forces, and have low melting points, are easily vaporized, soft, and good insulators. Ex: Sugar, Dry Ice Pictures of Solids: http: //ull. chemistry. uakron. edu/genobc/Chapter_06/


Crystalline Solids

Covalent Network: Diamond

Covalent Network: Graphite

http: //www. wwnorton. com/COLLEGE/chemistry/gilbert/tutorials/interface. asp? chapter=chapter_09&folder=phase_diagrams Tutorial on phase diagram with phase changes

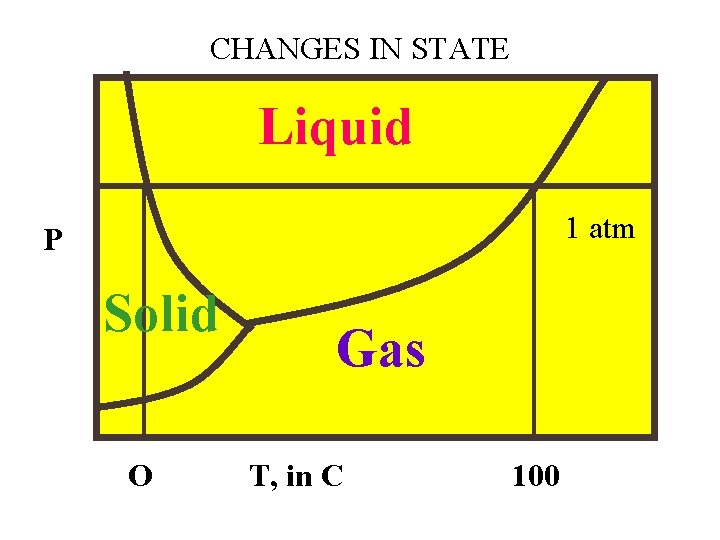
CHANGES IN STATE Liquid 1 atm P Solid O Gas T, in C 100


 Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Liquid to gas
Liquid to gas Mass of solid liquid and gas
Mass of solid liquid and gas Properties of solids liquids and gases
Properties of solids liquids and gases Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Venn diagram of states of matter
Venn diagram of states of matter Process of liquid to gas
Process of liquid to gas Adhesive force
Adhesive force Liquids and solids menu
Liquids and solids menu Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kesler science.com
Kesler science.com Red liquid element
Red liquid element How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Why is gas easier to compress than a liquid or a solid
Why is gas easier to compress than a liquid or a solid Filtration
Filtration Superplasticity
Superplasticity Liquid state of matter properties
Liquid state of matter properties Properties of platonic solids
Properties of platonic solids Strength property of matter
Strength property of matter Properties of network covalent solids
Properties of network covalent solids Elastic properties of solids
Elastic properties of solids Weight=density*volume
Weight=density*volume Bulk properties of solids
Bulk properties of solids Extensive vs intensive properties
Extensive vs intensive properties Physical property and chemical property
Physical property and chemical property Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Thang điểm glasgow
Thang điểm glasgow Chúa sống lại
Chúa sống lại