Properties of Liquids Properties of Liquids Definite volume















- Slides: 17

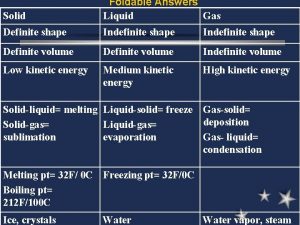
Properties of Liquids

Properties of Liquids • Definite volume • Indefinite shape • Particles are close together, but can move a little bit. Liquids can flow. • Density of liquids is much greater than gases. Ex: DH 2 Ol is 1250 X greater than DH 2 Og at 25 C. • Liquids can be compressed but change in volume is very slight & requires enormous pressure.

Viscosity • Liquids exhibit viscosity. • Viscosity = resistance to flow. • Viscosity depends on strength of intermolecular forces, sizes & shapes of molecules, and temperature. • The stronger the intermolecular forces, the higher the viscosity. • As temperature , viscosity .

Oil in an engine prevents direct metal to metal contact. Need a thin film of oil on bearing surfaces to prevent flaking of metal. If the oil is too thick, it won’t circulate at low temperatures. If the oil is too thin, it will lose film strength at high temperatures.

Where does the marble drop fastest? water Slowest? glycerol

Viscosity & Petroleum Drilling

Surface Tension • Particles at the surface of a liquid exist in an unbalanced environment. No attraction from above to balance attractions from below. • Net attractive force pulling down. • Surface seeks smallest possible area.


Surface Tension • Surface Tension = energy required to increase the surface area by a given amount. = measure of inward pull. • Strong intermolecular attractions High surface tension.



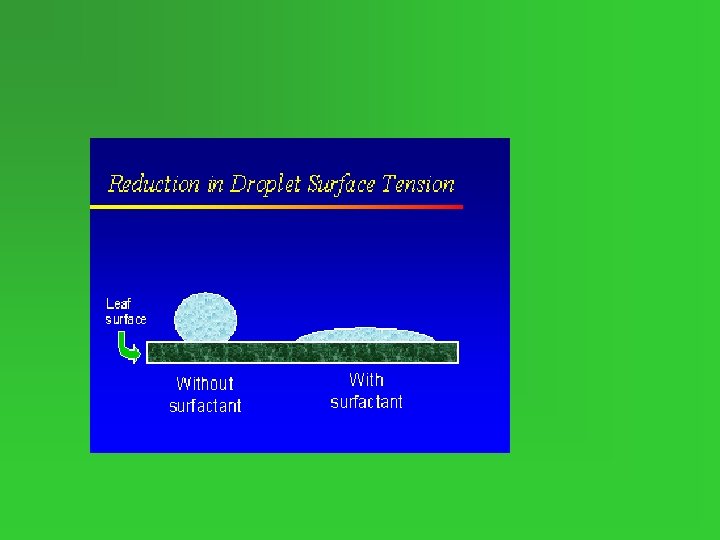
Surfactants • Compounds that lower the surface tension of water • Disrupt hydrogen bonds between H 2 O molecules. • See video


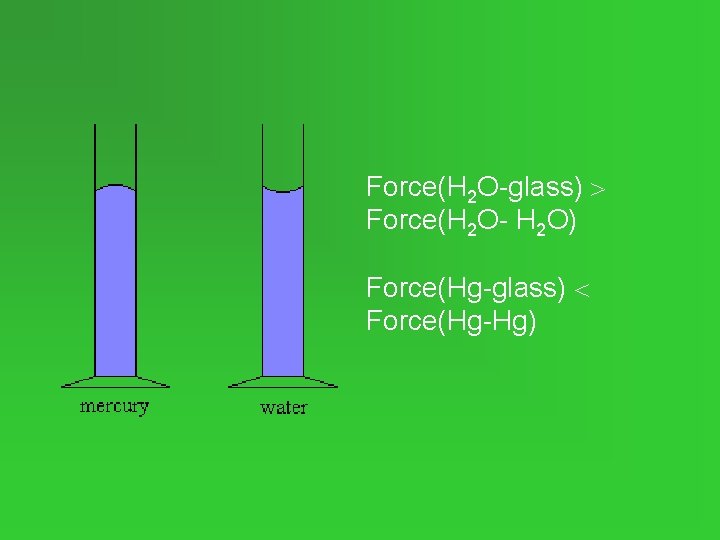
Capillary Action • Water forms a concave meniscus in a glass tube. • Attractive forces between water and glass > attractive forces between the water molecules. • Upward movement of a liquid in a narrow tube = capillary action.



Force(H 2 O-glass) Force(H 2 O- H 2 O) Force(Hg-glass) Force(Hg-Hg)
 Solid liquid gas plasma
Solid liquid gas plasma Is the volume of a liquid definite or indefinite
Is the volume of a liquid definite or indefinite Circuit training properties of definite integrals
Circuit training properties of definite integrals 20 examples of liquids
20 examples of liquids Liquid state of matter properties
Liquid state of matter properties Matter and its composition
Matter and its composition Buoyancyability
Buoyancyability Solid liquid and gas particles
Solid liquid and gas particles Stroke volume ejection fraction
Stroke volume ejection fraction What is large volume parenterals
What is large volume parenterals Solute
Solute Lung capacity
Lung capacity Stroke volume units
Stroke volume units Volume kerucut = .....x volume tabung *
Volume kerucut = .....x volume tabung * Properties of matter definition
Properties of matter definition Brain pop density
Brain pop density Definite arrangement
Definite arrangement Italian definite and indefinite articles chart
Italian definite and indefinite articles chart