LARGE VOLUME PARENTERALS CONTENTS INTRODUCTION FORMULATION PRODUCTION QUALITY



























- Slides: 31

LARGE VOLUME PARENTERALS

CONTENTS • INTRODUCTION • FORMULATION • PRODUCTION • QUALITY CONTROL TESTS • CONCLUSION • REFERENCES

INTRODUCTION • The USP provides the definition for large volume parenterals (LVP’s) “where used in the pharmacopeia, the designation Large Volume solution applies to an injection that is intended for intravenous use and is packaged in containers holding 100 ml or more”. • “LVP’s” means a terminally sterilized aqueous drug products packaged in a single dose container with a capacity of 100 ml or more and intended to be administered or used in man. • It includes IV infusions, irrigating solutions, peritoneal dialysates and blood collecting units with anticoagulant.

Formulations have been developed to: • Supply the water, electrolytes and simple carbohydrates needed by the body. • Act as vehicle for infusion of drugs that are compatible in the solution. • Supply nutritional requirements when the nutrients cannot be taken orally. • Provides solutions to correct acid-base balance in the body. • Promote diuresis when the body is retaining fluids. • Act as dialysing agents in patients with impaired kidney function.

FORMULATION Water for injection: • WFI is used as the main solvent in parenteral preparations. • Purified , deionised, water may be employed during the early phase of the manufacturing process. • The incoming water is adequately pretreated to ensure its uniformity and to promote quality. • WFI is the water purified by distillation or by reverse osmosis and contains no added substances.

• Inorganic salts used include the alkaline (Na and K) and alkaline earth (Ca and Mg) chlorides and alkaline phosphates. These salts provide necessary electrolytes and also used to adjust the isotonicity. • Carbohydrates such as Monosaccharides (dextrose and fructose), Disaccharides (e. g. sucrose and maltose ) Polysaccharides such as dextran. • Nitrogen containing substances such as aminoacids and protein hydrolysates. • Drugs such as lidocaine, theophylline, heparin, dopamine, and nitro glycerin are formulated in dextrose or saline diluents. • Acids (Hcl or acetic) and bases (Nao. H) are also used for p. H adjustment in the manufacture of LVP’s.

• Buffers: Many drugs require a certain p. H range to maintain the product stability. Drugs solubility may also be strongly dependent on the p. H of the solution. The product can be buffered to approach maximum or minimum solubility, whichever is desired. • Tonicity modifiers: Are used in many parenteral and products to maintain the same osmotic pressure as blood and to reduce tissue damage, irritation , pain of injection. • Antioxidants are also used in these formulation. • Eg: Ascorbic acid, Sodium bi sulfite.

PRODUCTION QUALITY AND STABILITY: • The quality of the starting materials and solutes is critical to the finished LVP’s product. • Presence of contaminants or degradates that effect finished product are considered. • Heat , light, moisture and air can adversely effect many of these materials. • The containers for these drug substances can also be imp factors in stability considerations.

RECEIVING: • A specific, isolated area is usually dedicated as a delivery point for the receipt of raw materials. All raw materials must be inspected, identified, documented and sampled in accordance with written procedures. • All raw materials should be issued to production on a first-in/firstout (FIFO) basis, the oldest materials should be used first. • Materials should be received covered or in closed containers. STORAGE/QUARANTINE AREAS: • All materials associated with the final drug products must be sampled, distributed to laboratory functions from the quarantine areas.

BATCH MIXING: • The majority of LVP’s are simple aqueous soln’s. The process for manufacturing simple LVP’s is straight forward, since the solutes are readily soluble in water. • The solutions mix tank is filled with WFI, and the weighted solutes are added with mixing to effect dissolution. The solution is assayed to confirm the acceptability of the solute concentrations. • The solution mix tanks are made of stainless steel and are fitted with agitators to provide a uniform concentration throughout the contents.

• Some solutions may require heat to effect dissolution of the solutes • eg: solutions that are very concentrated or solutions where solutes have low solubility. Jacketed mixing tanks are used in the manufacture of these types of solutions. • These tanks also have temperature monitoring devices to mointor the temperature of the solution. • Careful consideration must be given to the temperature and duration of heating to ensure minimal degradative effects on the solution. subsequent cooling may be needed to minimize degradation of less stable solutes.

IN-PROCESS ASSAYS: • In-process assays may be performed for one or more ions or other substances and adjustments in solute concentrations made as required before dilution to final prescribed volume • Analytical methods performed as in-process assays must be precise and accurate. FILTRATION: • Filtration may be defined as the separaton of undissolved particles from a liquid by passing a solution through a septum or porous medium that allows the liquid to pass but retains the particles.

• The filtration of liquids is one of the most important operations in pharmaceutical technology. Originally, liquid products were filtered to improve their clarity and pharmaceutical elegance. • The rate of filtration can be measured as the volume (mass) of fluid passing through the filter in a unit of time. • The driving force is the difference in pressure measured upstream and downstream of the filter. • Membrane filters, screen filters, cake filters, depth filters are used for this filtration process.

CLEANING PROCESS EQUIPMENT AND LINES • Water systems: Study of microbiology of water is imp, because water is used for cleansing the equipment before and after manufacture of parenterals. • Mostly gram-negative org’s like Coliform bacteria, pseudomonas, xanthomonas, flavobacterium are water born and these are killed by autoclaving. • All the parts of the equipment should be disassembled, sanitized, cleaned, thoroughly rinsed with water, dried , and inspected for leaks before reassembly.

CONTAINERS AND CLOSURES: • Glass containers: glass containers have been employed for LVP’s. Glasses are molded, often with graduations. Solid rubber stoppers are used for the container closure system. • Because of weight and fragility , the glass containers have been largely replaced by plastic. Glass is used only for sol’ns which are basically incompatible with plastic (e. g. lipid emulsions which may extract plasticizers). • Glass is very strong but the great concern is for glass particles, which may be sprayed around the area contaminating other bottles.

• Washed , cleaned glass containers should be held at a minimum of 70 o. C to supress microbial growth. • Removal of pyrogens from the containers are by subjecting them to a temp of 210 o. C for 3 -4 hours or 6500 C for 60 seconds. • Plastic containers: thermo plastic polymeric containers are used because, they are light in weight, non-breakable, with less amount of additives, less toxic, less reactive. • The materials include poly ethylene, poly propylene, PVC, nylon, poly styrene, teflon etc. Plastic Containers require screw caps fabricated of plastic as closures. • The plastic containers for parenteral use must be tested for toxicity.

FILLING: • Highly accurate fills are not necessary with LVP’s, filling is generally uncomplicated. • The USP recommends that parenterals with a vol of 50 ml or more contain a 2%excess for mobile liquids and a 3%excess for viscous liquids. • Flexible plastic containers are usually filled by volumetric displacement systems consisting of a cylinder and piston in draws a fixed volume of fluid that is then discharged into the container. The piston stroke , which is adjustable determines the volume.

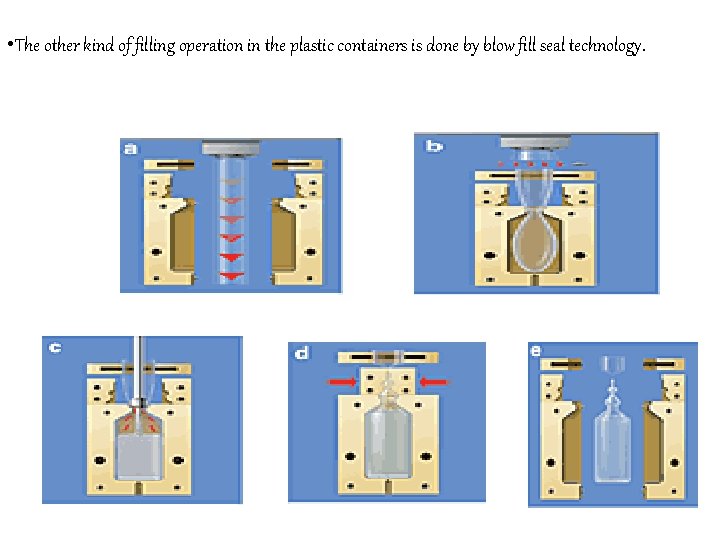
• The other kind of filling operation in the plastic containers is done by blow fill seal technology.

SEALING: • The sealing operation , which establishes the integrity of the container is typically varied as the design and composition of the container varies. • Glass containers require an elastomeric closure. The headspace above the fluid may be flushed with gas. Aluminium caps are then applied and crimped around the neck finish of the bottle. • Crimping is very critical phase of the sealing operation in that too much Crimping Force can result in a damaged seal, where as too little force will result in a weak seal.

PACKAGING: • The finished, filled containers are then packaged in corrugated card board cartons. These cartons are designed to facilitate shipping of finished products and to protect containers from physical damage. Cartons must be labelled with minimal product information (identification and storage conditions). TERMINAL STERILISATION: A process used to render the product sterile to a preferred sterility assurance level (SAL). • Moist and dry heat sterilisation processes are preferred, radiation and gaseous sterilisation are rarely employed.

LABELLING: • labelling of the LVP product must conform to the many USP and FDA requirements. • There are labels which are applied to individual containers as well as to the cartons used to package the containers for shipping and distribution. • The product labeling contains information detailing solution composition, p. H, batch number, and expiration dating period of the product. Storage requirements and specific precaution statements are also included. • For certain types of containers , such as glass or semirigid plastic containers , preprinted paper labels are applied to the container with adhesive.

QUALITY CONTROL: The QC unit is responsible for reviewing the batch history and performing the release testing required to clear the product for shipment to users. Quality control of parenterals: • Leaker test/container or closure integrity test: • This is done whether the contents may leak to outside and spoil the package, when micro-organisms or other contaminants may enter. • Changes in temp during storage cause expansion and contraction of glass/plastic and contents will accentuate passageway exists, even of microscopic size. interchange if

• The sealed containers are dipped in coloured sol’n of 0. 5%-1% methylene blue and vaccume applied, the sol’n inside the leaked container shows blue colour. • Particulate evaluation/clarity testing: • The parenteral preparations should be free from particulate matter in the range of 30 -40 micrometers and larger size particles. • USP states that all containers should be visually inspected for visible particles and if present they are discarded.

• In LVP’s the permitted limits of particulate matter is maximum of 50 particles of 10 micrometer and larger per ml. and 5 particles of 25 micrometer and larger per ml. and no particle should have a size of 50 micrometer or more. • Clarity is tested by visual inspection of containers under light and viewed against a black and white background. • Instrumental methods of evaluation is based on the principles of light scattering, light absorption and electrical resistance which are used to count particle and particle size distribution. • Coulter-counter method and filtration method are used for monitoring particulate matter.

• Pyrogen testing: • pyrogens are the metabolic products of gm-ve micro-org’s. pyrogens are lipopolysaccharides and are thermo stable. • This testing is done by 2 ways: • In vivo testing • In vitro testing • In vivo testing: The test involves the measurement of the rise in body temp of rabbit following IV injection of a sterile sol’n of a substance being examined.

• The soln under test is injected into 3 healthy rabbits slowly through an ear vein in a vol of 0. 5 to 10 ml/kg body weight, record the temp of each rabbit in an interval of 30 min for 3 hours after the injection. • If the response of any rabbit is 0. 6 or more , or if the sum of the responses of the rabbits exceeds 1. 4, continue the test using 5 other rabbits, if not more than 3 of 8 rabbits show individual responses of 0. 6 or more, and if the sum of the responses of the group of 8 rabbits does not exceed 3. 7, then the preparation passes the test.

• Invitro testing/LALtest: • LAL is cell lysate of blood cells of horse shoe shaped crab. Blood cells of crab are called as amoebocytes as they do not have perfect shape. • Soln of the preparation is made with WFI and LAL reagent is added to it , then incubate it at 370 C for 1 hour-gelly consistency indicates presence of pyrogens and no gel formation indicates absence of pyrogens.

• Sterility testing/microbiological tests: Sterility testing is done to know the sterility of the sterilised material. The medium selected for culturing should extremely support the growth of the microbes. • The protocol contains +ve control to check the ability of the medium to support growth and –ve control to ensure sterility of the media. • Safety testing: This test is essential particularly for biological products to provide additional assurance that the product does not have unexpected toxic properties.

CONCLUSION • Finally it could be concluded that Parenteral dosage forms differ from all other drug dosage forms because they are injected directly into body tissues through primary protective systems of human body the skin and mucous membranes.

REFERENCES • Pharmaceutical dosage forms: parenteral medications volume 1 and volume 2 by Kenneth E. Avis, Herbert A. Lieberman and Leon Lachman. • Leo. F. blow/ fill/ seal aseptic technology

THANK YOU