definite volume definite shape regular geometric pattern crystalline







- Slides: 14

definite volume definite shape regular geometric pattern crystalline structure vibrate in a fixed location strong forces of attraction Molecules farther apart definite volume no definite shape intermolecular forces noncompressible Molecules far apart no definite shape no definite volume no force of attractions Compressible Completely fills its container

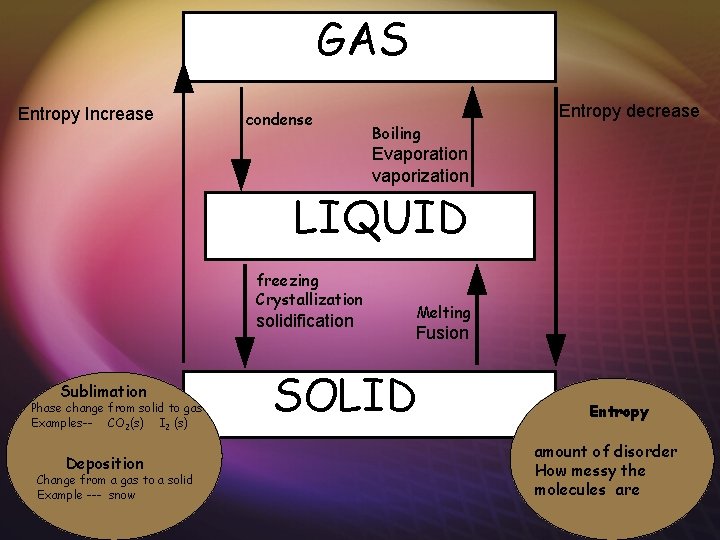
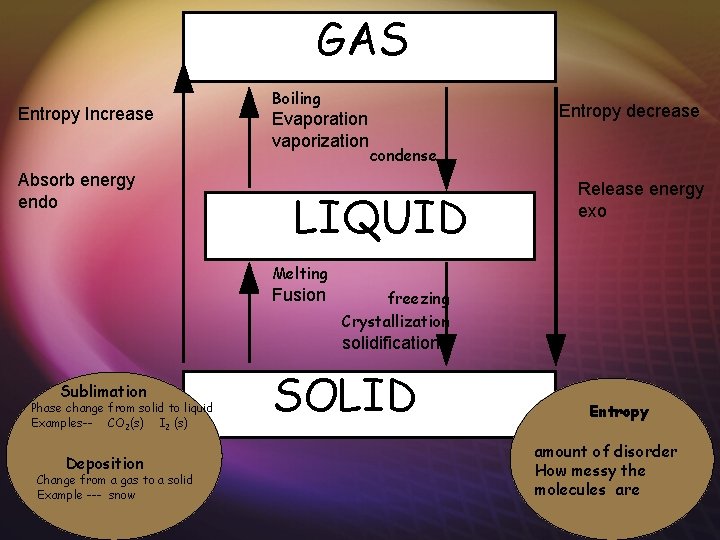
GAS Entropy Increase condense Entropy decrease Boiling Evaporation vaporization LIQUID freezing Crystallization solidification Sublimation Phase change from solid to gas Examples-- CO 2(s) I 2 (s) Deposition Change from a gas to a solid Example --- snow Melting Fusion SOLID Entropy amount of disorder How messy the molecules are

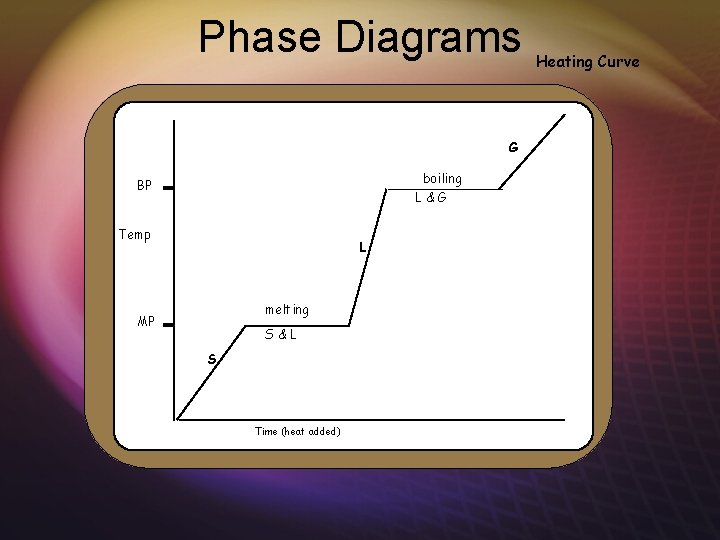
Phase Diagrams boiling L&G BP Temp L melting MP S&L S Time (heat added) Heating Curve G boiling

Melting Point the temperature at which the solid and liquid phase of a substance are in equilibrium --also freezing point Boiling Point the temperature at which the liquid and gas phase of a substance are in equilibrium --- also condensation point Water 100 o. C Water 0 o. C

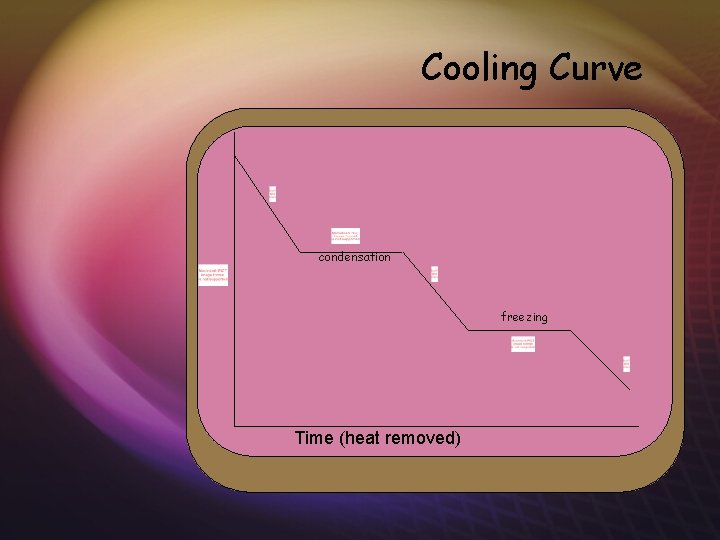
Cooling Curve condensation freezing Time (heat removed)

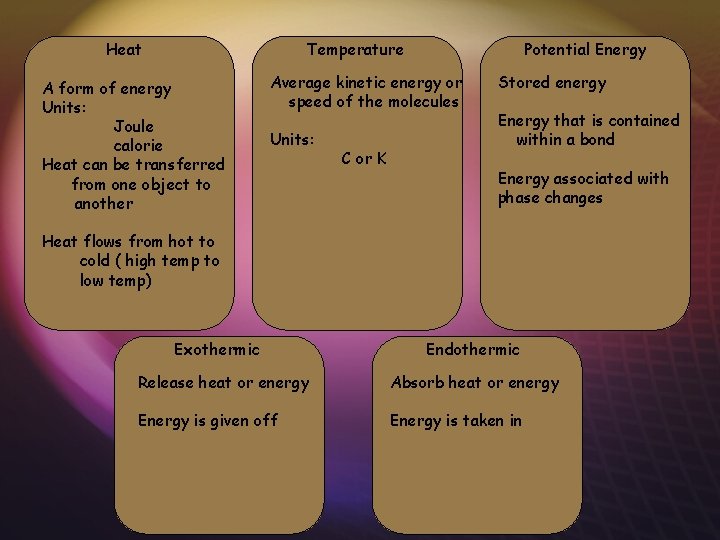
Heat Temperature A form of energy Units: Joule calorie Heat can be transferred from one object to another Potential Energy Average kinetic energy or speed of the molecules Units: C or K Stored energy Energy that is contained within a bond Energy associated with phase changes Heat flows from hot to cold ( high temp to low temp) Exothermic Endothermic Release heat or energy Absorb heat or energy Energy is given off Energy is taken in

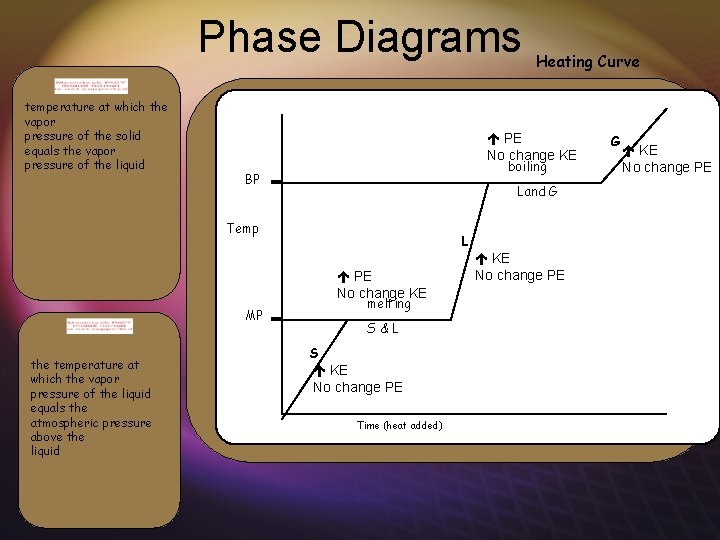
Phase Diagrams temperature at which the vapor pressure of the solid equals the vapor pressure of the liquid PE No change KE boiling BP Land G Temp L PE No change KE melting MP the temperature at which the vapor pressure of the liquid equals the atmospheric pressure above the liquid Heating Curve S&L S KE No change PE Time (heat added) KE No change PE G KE No change PE

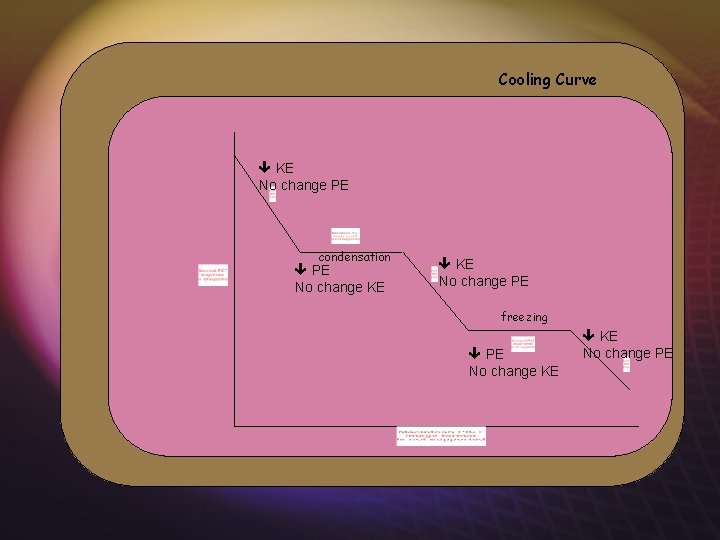
Cooling Curve KE No change PE condensation PE No change KE No change PE freezing PE No change KE No change PE

GAS Entropy Increase Absorb energy endo Boiling Evaporation vaporization Entropy decrease condense LIQUID Release energy exo Melting Fusion freezing Crystallization solidification Sublimation Phase change from solid to liquid Examples-- CO 2(s) I 2 (s) Deposition Change from a gas to a solid Example --- snow SOLID Entropy amount of disorder How messy the molecules are

Vapor Pressure • The pressure vapor exerts over it’s liquid on a closed system. • The more liquid that evaporates, the higher the vapor pressure! • Vapor pressure is temp. dependent. Up temp = up Vapor Pressure • If no cap- open system- VP= atm pressure


Boiling • Boiling occurs when atmospheric pressure = vapor pressure. • Increase atmospheric pressure you increase boiling point. • Stronger intermolecular forces = higher BP • A normal boiling point- temperature when something boils at standard pressure 101. 3 k. Pa.


The point at which all 3 states of matter can exist at the same time Occurs at the end of the l/g line Can not distinguish between a liquid and a gas Positive: the solid is more dense than the liquid (sink) Negative: the solid is less dense than the liquid (float)