Liquids Properties of liquids are similar to solids





- Slides: 6

Liquids Properties of liquids are similar to solids’, and way different from gas properties • density • compressibilities • enthalpy changes • attractive forces

INTERMOLECULAR ATTRACTIVE FORCES between molecules (liquids & solids) • Forces that act between molecules (or atoms) keeping them in one phase, liquid • A sample of a liquid will remain in that phase until IAFs are overcome, usually due to an increase in temperature or pressure • For IAFs, you focus on many molecules in a sample, once you have determined if you have polar or non-polar bonds between the atoms that make up the molecules • Remember, bond determination occurs due to electronegativity differences between two atoms (0. 0 – 0. 4 – non-polar covalent bond, 0. 4 – 1. 9 polar covalent bond, > 1. 9 – ionic bonds) • IAF determination occurs when you look at the interaction of molecules.

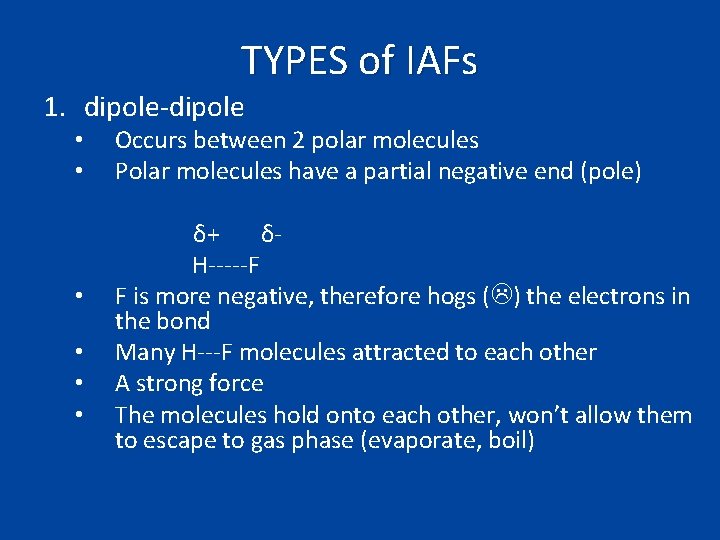
TYPES of IAFs 1. dipole-dipole • • • Occurs between 2 polar molecules Polar molecules have a partial negative end (pole) δ+ δH-----F F is more negative, therefore hogs ( ) the electrons in the bond Many H---F molecules attracted to each other A strong force The molecules hold onto each other, won’t allow them to escape to gas phase (evaporate, boil)

2. Hydrogen Bonding • • • A force between molecules NOT a bond between atoms A special type of dipole-dipole Between H and N, O, or F The STRONGEST IAF!!! Molecules with hydrogen bonding have the highest melting and boiling point of covalent, molecular compounds --- why? δ+ H δO δ+ H Each H—O bond is polar Eneg difference is 1. 4 Oxygen is the hog

3. Ion-dipole forces • Between ion and greatly polar molecule, like • Accounts for solubility • Ionics in H 20 – solutions • Na—Cl soluble? Yes, why? - Cl ion is attracted to δ+ H in H 20 + Na ion is attracted to δ- O in H 20 • If the attraction is strong enough, the compound will be soluble. Is Pb. SO 4 soluble?

4. London dispersion forces • Also called van der Waals forces • Among non-polar molecules • Weakest IAF • Minimal attraction • H 2, Cl 2, CO 2 at room temperature are gases, overcome IAFs