Water Notes Water Molecule Water is a POLAR




- Slides: 8

Water Notes

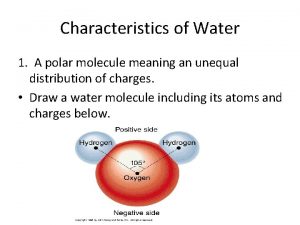
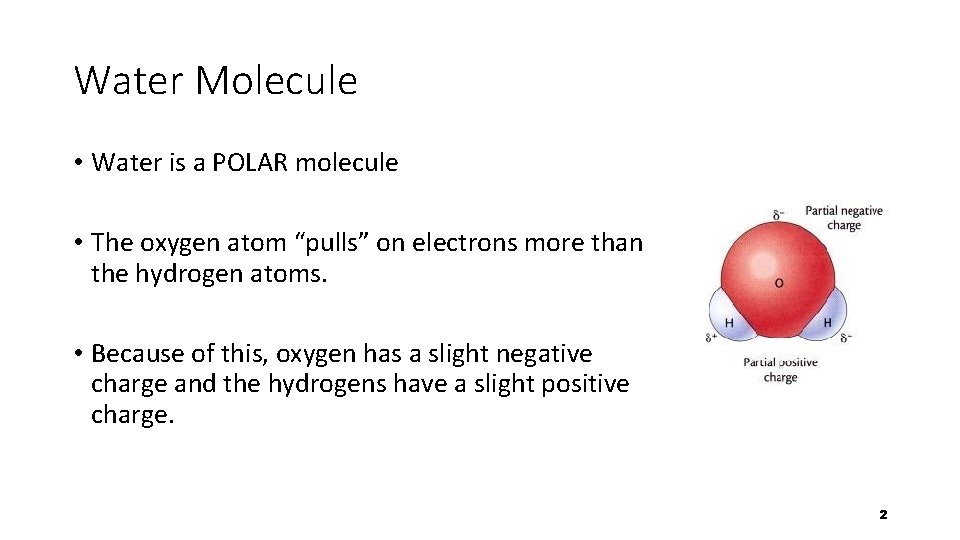
Water Molecule • Water is a POLAR molecule • The oxygen atom “pulls” on electrons more than the hydrogen atoms. • Because of this, oxygen has a slight negative charge and the hydrogens have a slight positive charge. 2

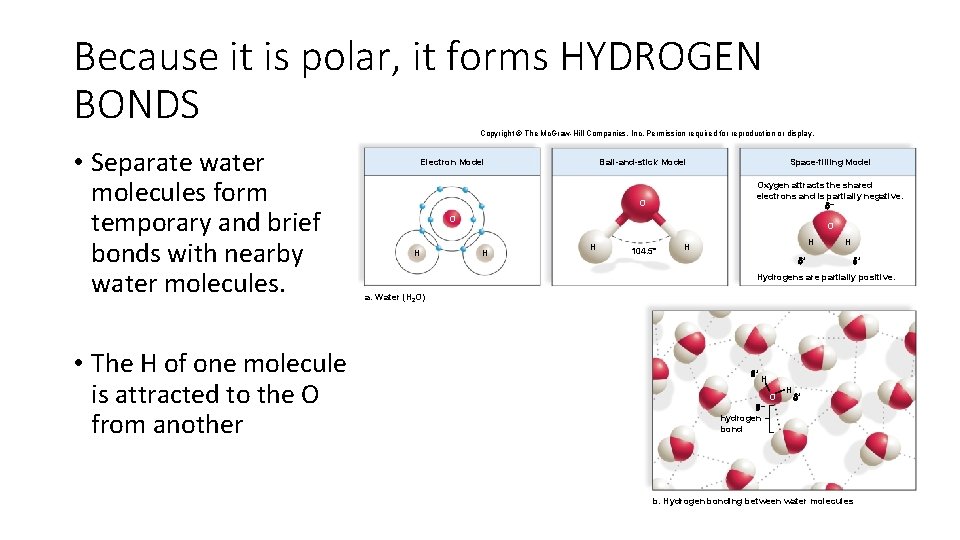
Because it is polar, it forms HYDROGEN BONDS Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. • Separate water molecules form temporary and brief bonds with nearby water molecules. • The H of one molecule is attracted to the O from another Electron Model Ball-and-stick Model Space-filling Model O Oxygen attracts the shared electrons and is partially negative. – O H H 104. 5° H H + Hydrogens are partially positive. a. Water (H 2 O) + H – O H + hydrogen bond b. Hydrogen bonding between water molecules

Properties of Water 1 and 2: Cohesion & Adhesion • Cohesion: • Hydrogen bonds hold water molecules tightly together • i. e. allows water to flow freely without molecules separating. • Adhesion: • Hydrogen bonds for between water and other polar materials • Allow water be drawn many meters up a tree in a tubular vessel 4

Property 3: High Surface Tension • High Surface Tension • Water molecules at surface hold more tightly than below surface • Amounts to an invisible “skin” on water surface • Allows small nonpolar objects (like water strider) to sit on top of water

Property 4: Capillary Action • Because of cohesion and adhesion, water has the ability to move through narrow spaces. Water evaporates, pulling the water column from the roots to the leaves. H 2 O • This is why water seeps through a paper towel, and how it moves against gravity to the tops of trees Water molecules cling together and adhere to sides of vessels in stems. Water enters a plant at root cells. H 2 O Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. 6

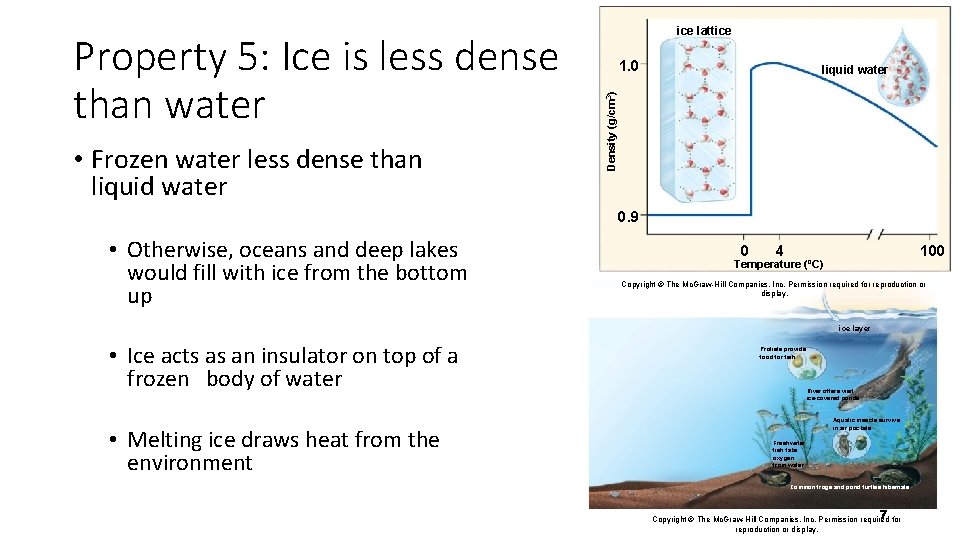
• Frozen water less dense than liquid water 1. 0 liquid water Density (g/cm 3) Property 5: Ice is less dense than water ice lattice 0. 9 • Otherwise, oceans and deep lakes would fill with ice from the bottom up 0 4 100 Temperature (ºC) Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. ice layer • Ice acts as an insulator on top of a frozen body of water • Melting ice draws heat from the environment Protists provide food for fish. River otters visit ice-covered ponds. Aquatic insects survive in air pockets. Freshwater fish take oxygen from water. Common frogs and pond turtles hibernate. 7 for Copyright © The Mc. Graw-Hill Companies, Inc. Permission required reproduction or display.

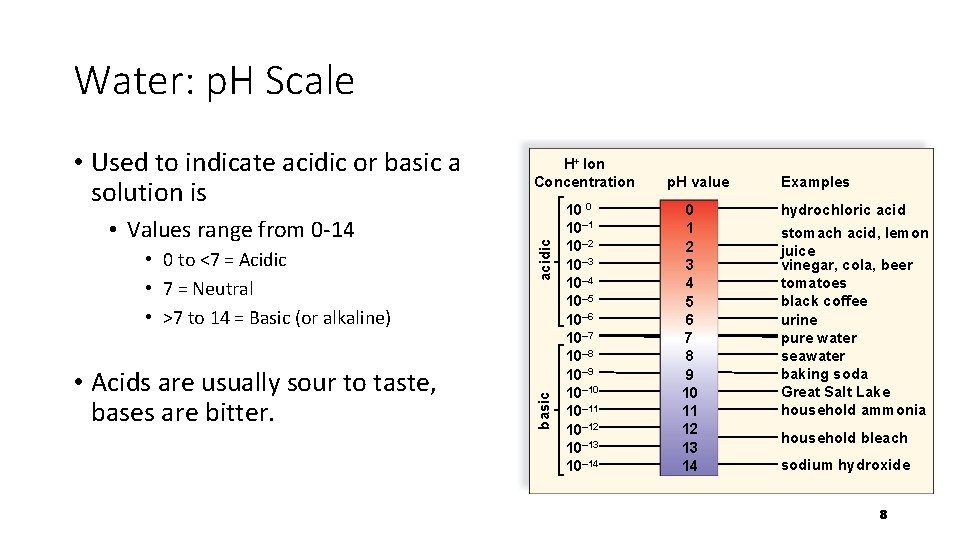
Water: p. H Scale • 0 to <7 = Acidic • 7 = Neutral • >7 to 14 = Basic (or alkaline) • Acids are usually sour to taste, bases are bitter. acidic • Values range from 0 -14 H+ Ion Concentration basic • Used to indicate acidic or basic a solution is 10 0 10– 1 10– 2 10– 3 10– 4 10– 5 10– 6 10– 7 10– 8 10– 9 10– 10 10– 11 10– 12 10– 13 10– 14 p. H value 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Examples hydrochloric acid stomach acid, lemon juice vinegar, cola, beer tomatoes black coffee urine pure water seawater baking soda Great Salt Lake household ammonia household bleach sodium hydroxide 8