Properties of Water ETO Properties of Water Polar

















- Slides: 31

Properties of Water ETO

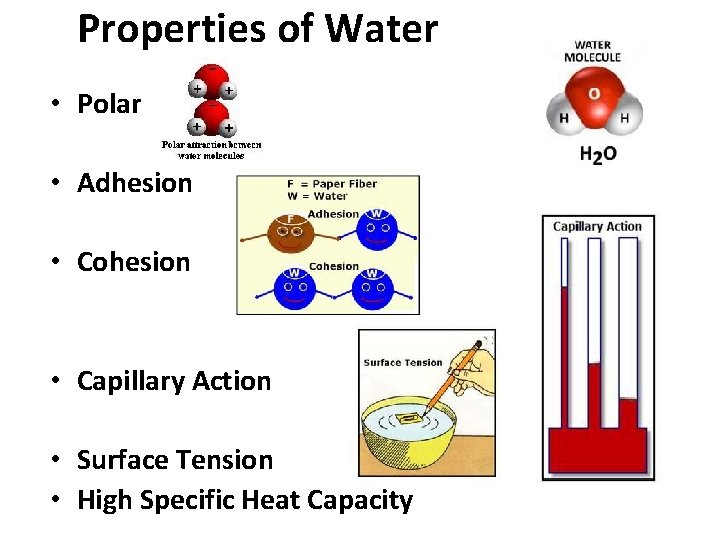
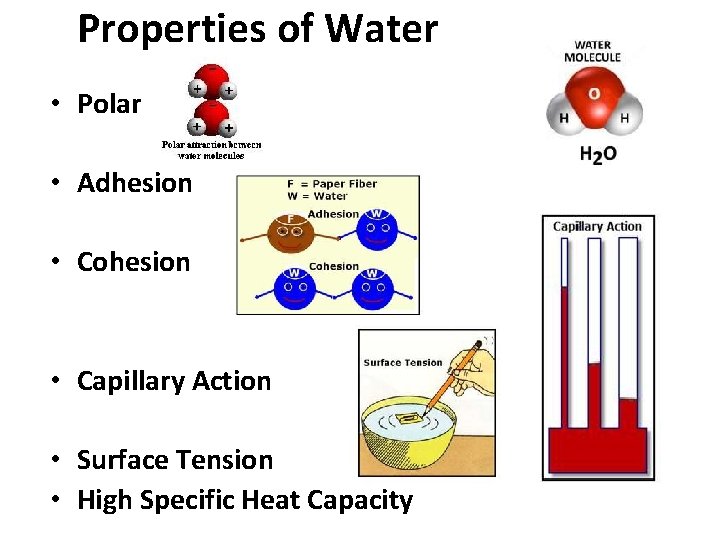
Properties of Water • Polar • Adhesion • Cohesion • Capillary Action • Surface Tension • High Specific Heat Capacity

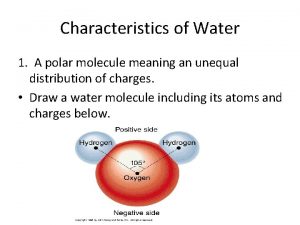
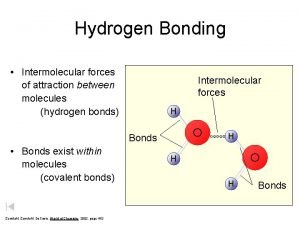
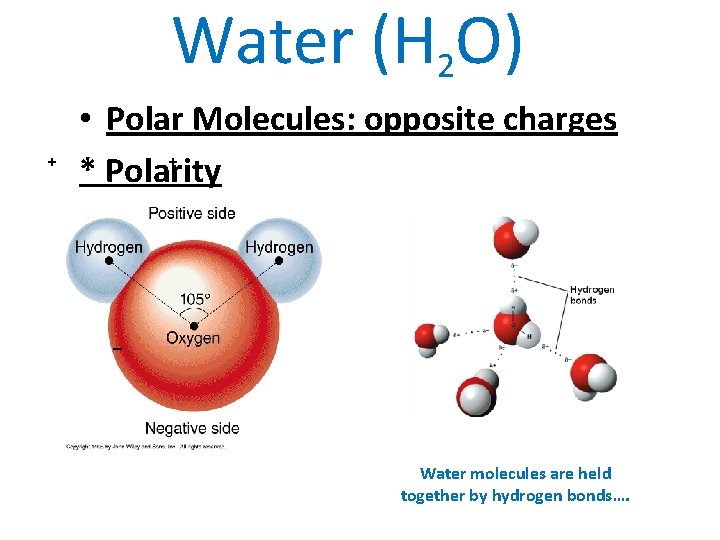
Water (H 2 O) + • Polar Molecules: opposite charges + * Polarity _ Water molecules are held together by hydrogen bonds….

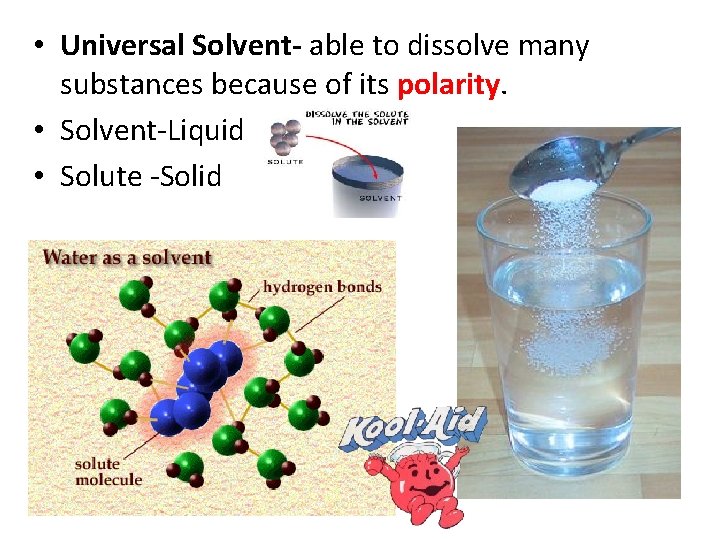
• Universal Solvent- able to dissolve many substances because of its polarity. • Solvent-Liquid • Solute -Solid

Non- Polar WAXY doesn’t mix… Lipids are non-polar Cuticle

Is waters reaction Polar or Non-Polar + - - + • Polar molecules will react will other polar molecules. (Ex- Paper and Water. ) • * they will show an attraction. + - • Polar molecules will not react with nonpolar molecules. (Ex: Oil and Water) • * they will not show an attraction.

Polar or Non-Polar

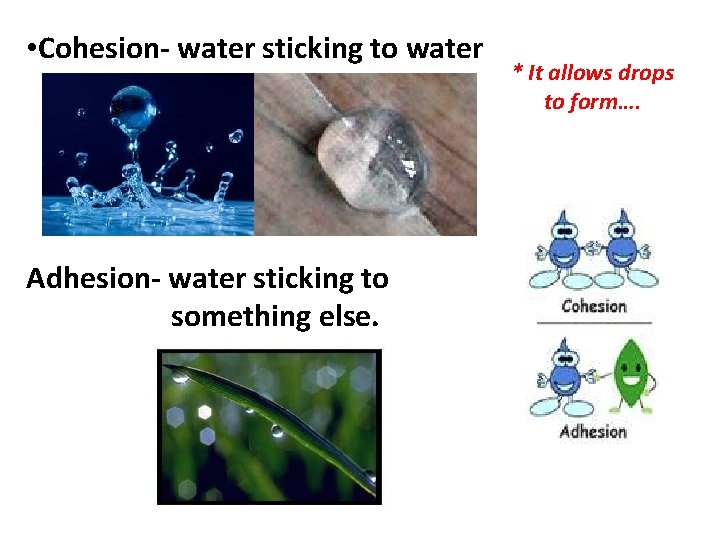
• Cohesion- water sticking to water Adhesion- water sticking to something else. * It allows drops to form….

ADHESION COHESION

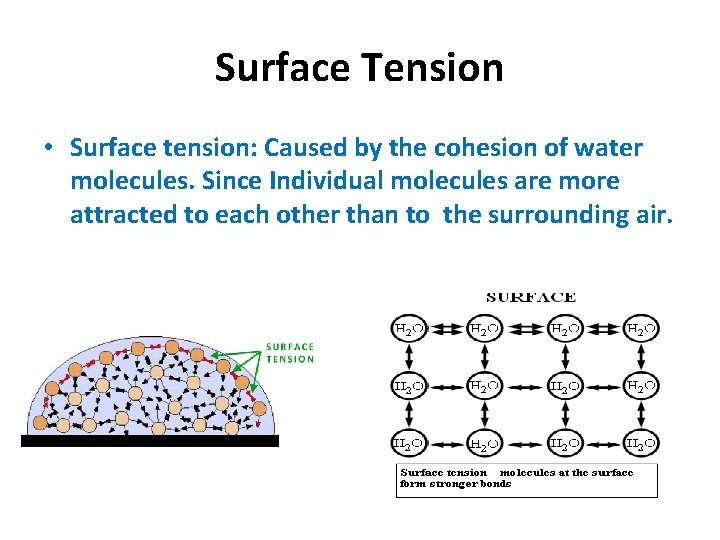
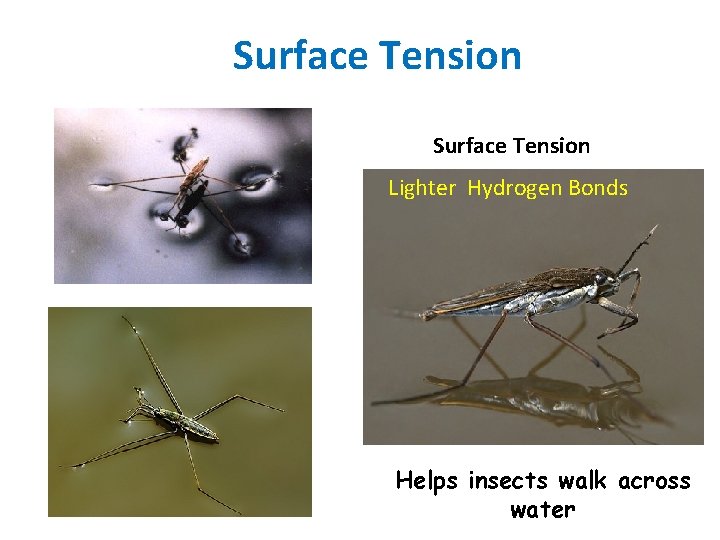
Surface Tension • Surface tension: Caused by the cohesion of water molecules. Since Individual molecules are more attracted to each other than to the surrounding air.

Surface tension- water forms a film on the surface.

Surface Tension Lighter Hydrogen Bonds Helps insects walk across water

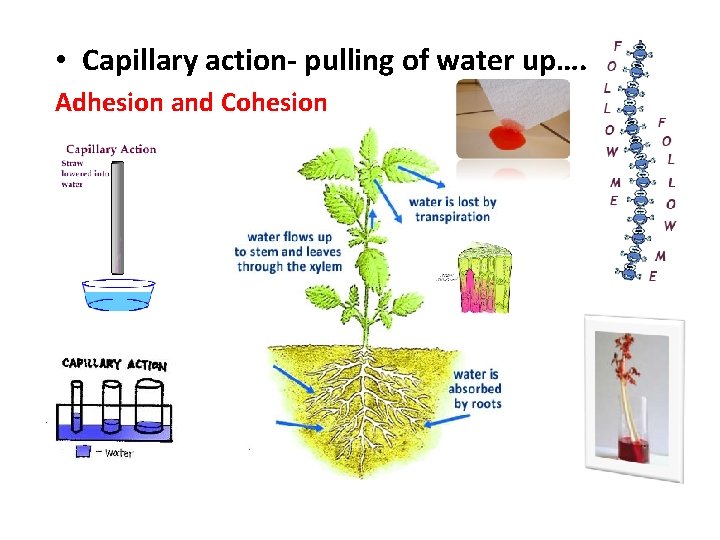
• Capillary action- pulling of water up…. Adhesion and Cohesion

Capillary Action in Carnations Adhesion and Cohesion

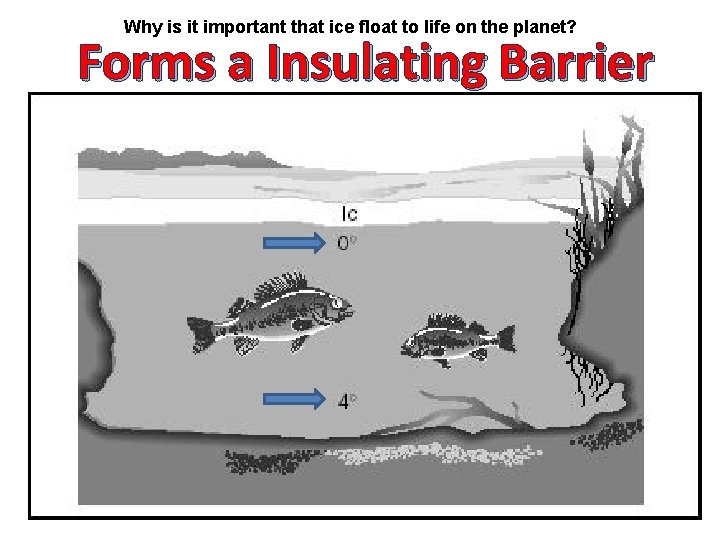
As water freezes, its molecules spread apart making frozen water less dense than liquid water. * So it floats……. LATTICE http: //www. youtube. com/watch? v=s. Qd. Ltt. Uh_b 0

Water is Less Dense as a Solid • Ice is less dense as a solid than a liquid …. That’s why ice floats LATTICE

Why is it important that ice float to life on the planet? Forms a Insulating Barrier

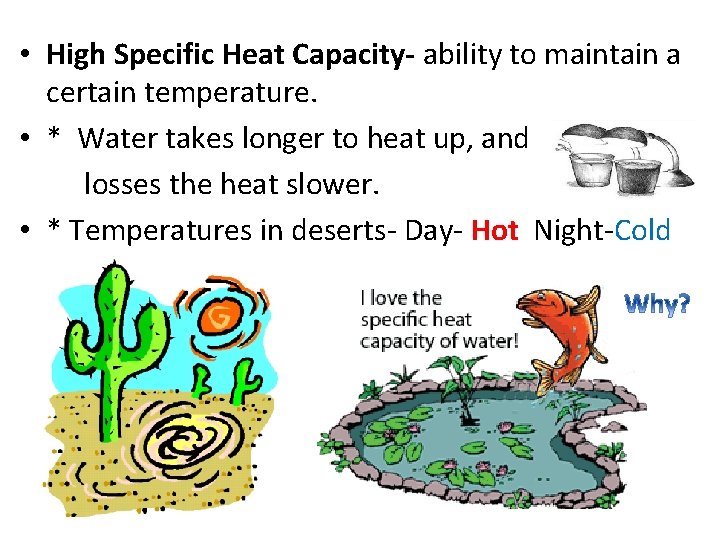
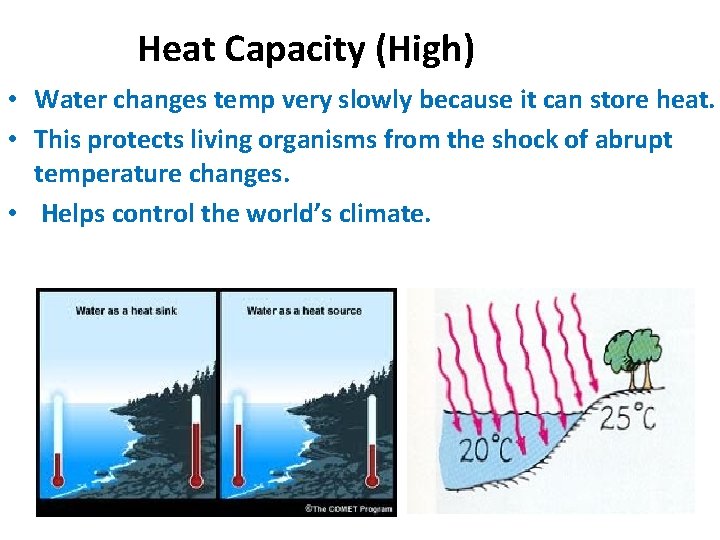
• High Specific Heat Capacity- ability to maintain a certain temperature. • * Water takes longer to heat up, and losses the heat slower. • * Temperatures in deserts- Day- Hot Night-Cold

Heat Capacity (High) • Water changes temp very slowly because it can store heat. • This protects living organisms from the shock of abrupt temperature changes. • Helps control the world’s climate.

1. Water dissolves many substances. This occurs because water has • A. surface tension • B. polarity • C. specific heat • D. cohesion

2. When the cells of most organisms freeze, they burst. Which property of water causes this to occur? A. Water is a universal solvent. B. Water changes temperature rapidly. C. Water is less dense as a solid than as a liquid. D. Water is a nonpolar molecule.

3. Some adult insects are unable to swim but are able to walk on top of water. What characteristic of water enables these insects to walk on top of water? • A. p. H • B. surface tension • C. solvent properties • D. atomic bonds

4. When water dissolves a substance, weak charges carried by water molecules attract the substance’s oppositely charged atoms and pull them away from their molecules. This is a function of which property of water? A. p. H B. polarity C. cohesion D. surface tension

5. Ice floats on a lake. This characteristic of water is responsible for — A. suffocation of aquatic organisms B. mixing a lake’s thermal layers C. preventing a lake from freezing solid D. altering migration patterns of fish

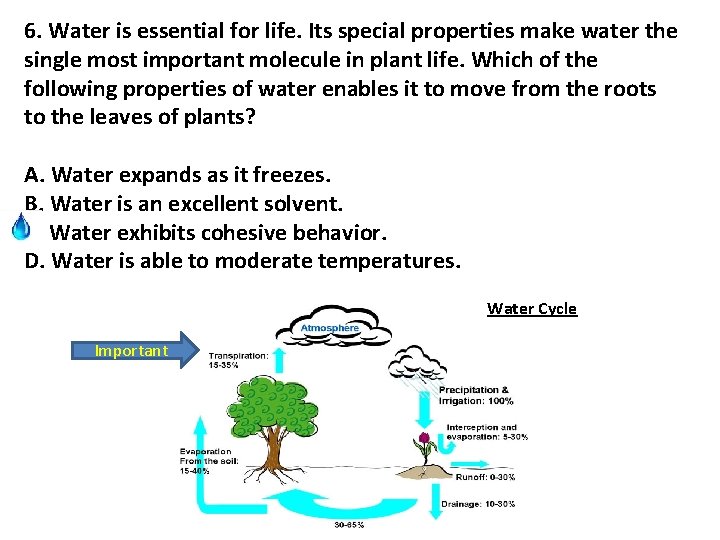
6. Water is essential for life. Its special properties make water the single most important molecule in plant life. Which of the following properties of water enables it to move from the roots to the leaves of plants? A. Water expands as it freezes. B. Water is an excellent solvent. C. Water exhibits cohesive behavior. D. Water is able to moderate temperatures. Water Cycle Important

7. Large bodies of water, such as lakes and oceans, do not quickly fluctuate in temperature. What is the reason for this phenomenon? • A. Water is an acid. • B. Water is a versatile solvent. • C. Water has a high heat capacity. • D. Water acts as a buffer.

8. Why does ice stay at the top of oceans instead of sinking to the bottom? • • A. Ice is colder than liquid water. B. Ice is less dense than liquid water. C. Ice is more dense than liquid water. D. Ice is warmer than liquid water. Forms a Insulating Barrier

10. A scientist believes that a factory has been dumping acid into a local river. To test this hypothesis, which property of water should the scientist monitor? • A. p. H • B. density • C. polarity • D. temperature

Properties of Water • Polar • Adhesion • Cohesion • Capillary Action • Surface Tension • High Specific Heat Capacity

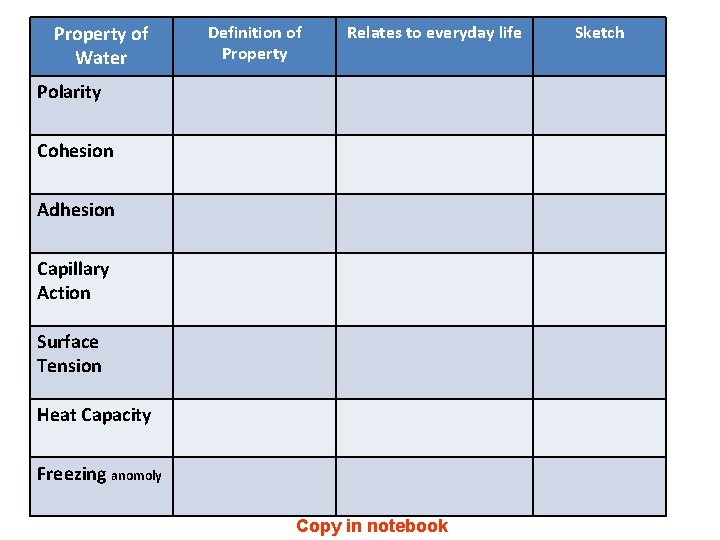
Property of Water Definition of Property Relates to everyday life Polarity Cohesion Adhesion Capillary Action Surface Tension Heat Capacity Freezing anomoly Copy in notebook Sketch

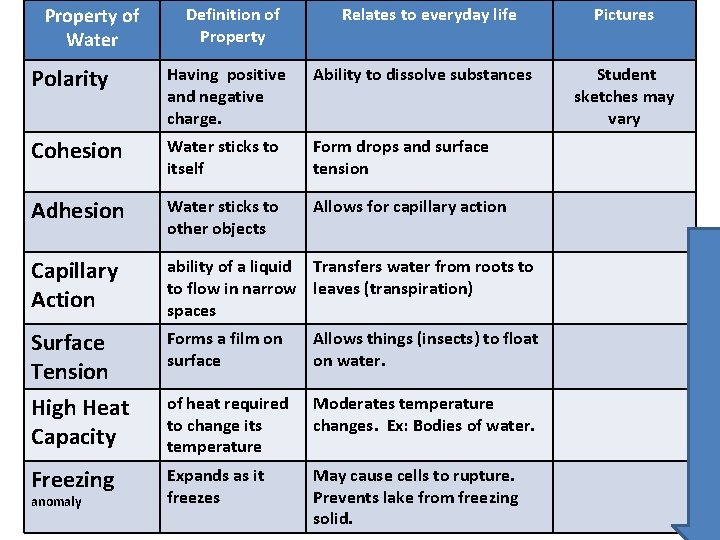
Property of Water Definition of Property Relates to everyday life Polarity Having positive and negative charge. Ability to dissolve substances Cohesion Water sticks to itself Form drops and surface tension Adhesion Water sticks to other objects Allows for capillary action Capillary Action ability of a liquid Transfers water from roots to to flow in narrow leaves (transpiration) spaces Surface Tension High Heat Capacity Forms a film on surface Allows things (insects) to float on water. of heat required to change its temperature Moderates temperature changes. Ex: Bodies of water. Freezing Expands as it freezes May cause cells to rupture. Prevents lake from freezing solid. anomaly Pictures Student sketches may vary
 Ano ang layunin sa paglalaro ng bugtong
Ano ang layunin sa paglalaro ng bugtong Nomenclatura óxidos
Nomenclatura óxidos Tetróxido de triferro
Tetróxido de triferro Tetroxido de triferro
Tetroxido de triferro Ethylene oxide fumigation
Ethylene oxide fumigation Pacific bio labs
Pacific bio labs Eto dadeschools
Eto dadeschools Contoh eto
Contoh eto Tkbf könyvtár
Tkbf könyvtár Properties of water polarity
Properties of water polarity Water and water and water water
Water and water and water water Water is polar or nonpolar
Water is polar or nonpolar No polar
No polar Polar and non polar amino acids
Polar and non polar amino acids Polar attractions are ...
Polar attractions are ... Polar and non polar dielectrics
Polar and non polar dielectrics Enlace dativo
Enlace dativo Is wax paper polar or nonpolar
Is wax paper polar or nonpolar Are water molecules polar
Are water molecules polar Polar molecule meaning
Polar molecule meaning Is water a covalent bond
Is water a covalent bond Zumdahl chemistry
Zumdahl chemistry Covalent bond
Covalent bond Polar covalent bond in water molecule
Polar covalent bond in water molecule Polar molecule
Polar molecule Polar covalent bond
Polar covalent bond Extensive vs intensive properties
Extensive vs intensive properties Chemical property definition
Chemical property definition Water concept map
Water concept map Properties of water ap bio
Properties of water ap bio Physical properties of sea water
Physical properties of sea water Properties of water clipart
Properties of water clipart