2 5 PolarPolar Attractions 2 5 1 Shape




- Slides: 10

2. 5 Polar-Polar Attractions 2. 5. 1. Shape and Polarity LO: I understand how the shape of a molecule can affect it’s polarity.

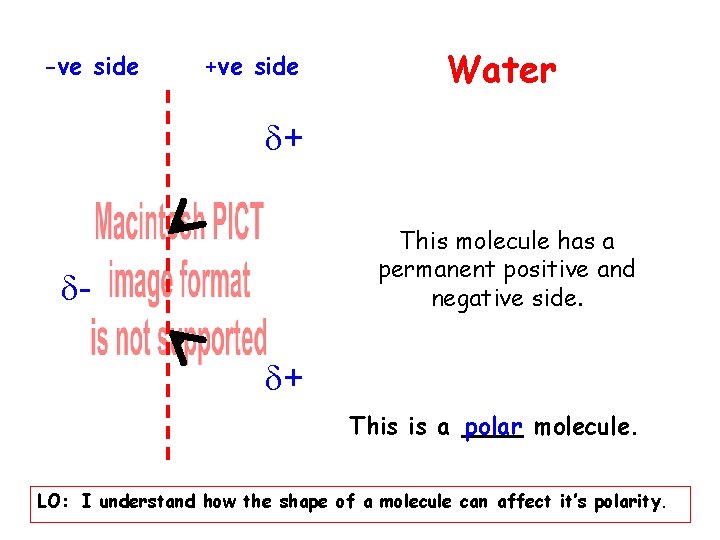
-ve side +ve side Water + This molecule has a permanent positive and negative side. + This is a polar molecule. LO: I understand how the shape of a molecule can affect it’s polarity.

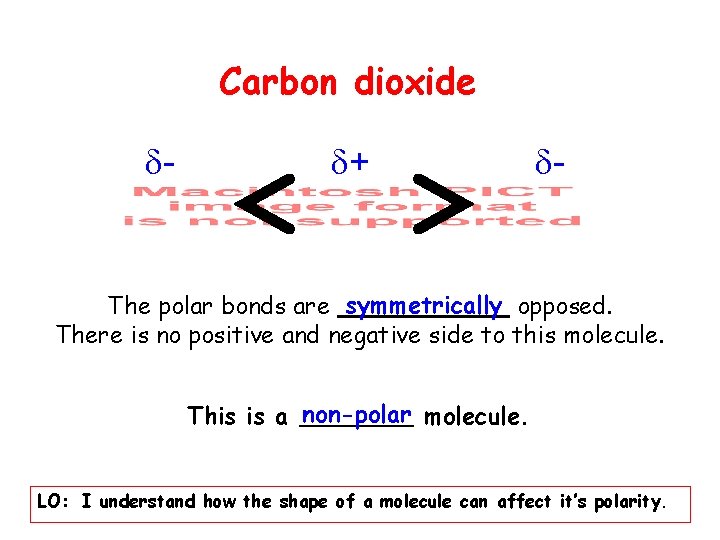
Carbon dioxide - + - The polar bonds are symmetrically opposed. There is no positive and negative side to this molecule. This is a non-polar molecule. LO: I understand how the shape of a molecule can affect it’s polarity.

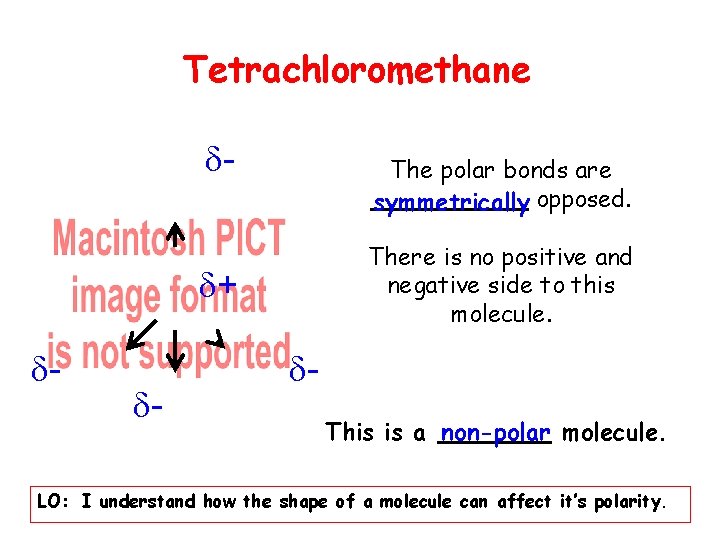
Tetrachloromethane - The polar bonds are symmetrically opposed. There is no positive and negative side to this molecule. + - - This is a non-polar molecule. LO: I understand how the shape of a molecule can affect it’s polarity.

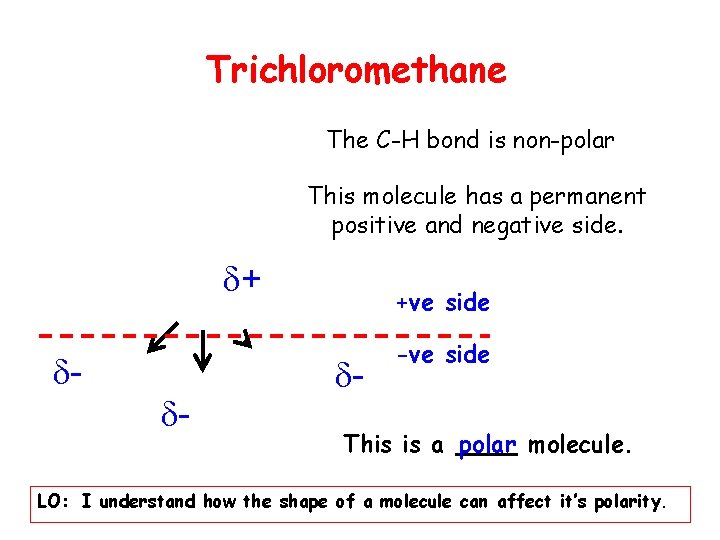
Trichloromethane The C-H bond is non-polar This molecule has a permanent positive and negative side. + - - +ve side - -ve side This is a polar molecule. LO: I understand how the shape of a molecule can affect it’s polarity.

2. 5. 2 Intermolecular Attractions and Solubilities LO: I understand the intermolecular forces between polar molecules and their affect on solubility.

Polar-polar attractions are intermolecular forces of attraction between molecules with a permanent dipole moment. In small molecules: polar-polar attractions > van der Waals forces. In large molecules: van der Waals forces > polar-polar attractions. LO: I understand the intermolecular forces between polar molecules and their affect on solubility.

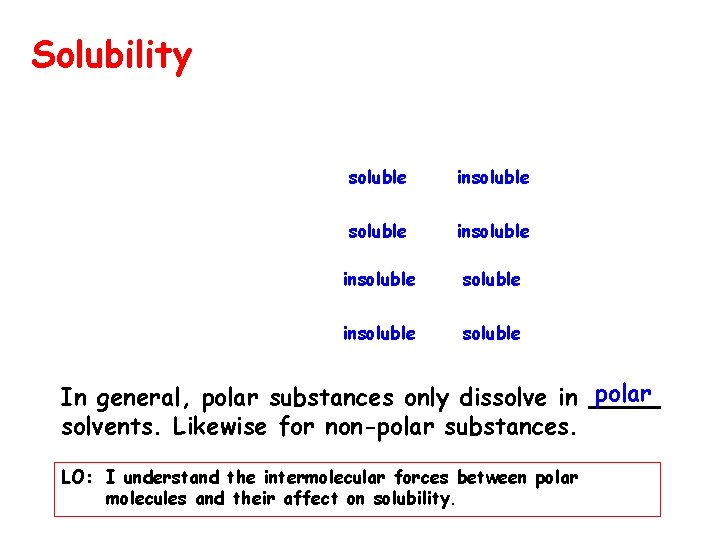
Solubility soluble insoluble soluble In general, polar substances only dissolve in polar. solvents. Likewise for non-polar substances. LO: I understand the intermolecular forces between polar molecules and their affect on solubility.

2. 5. 3 Comparing Boiling Points LO: I understand the affect polarity has on the boiling point of a substance.

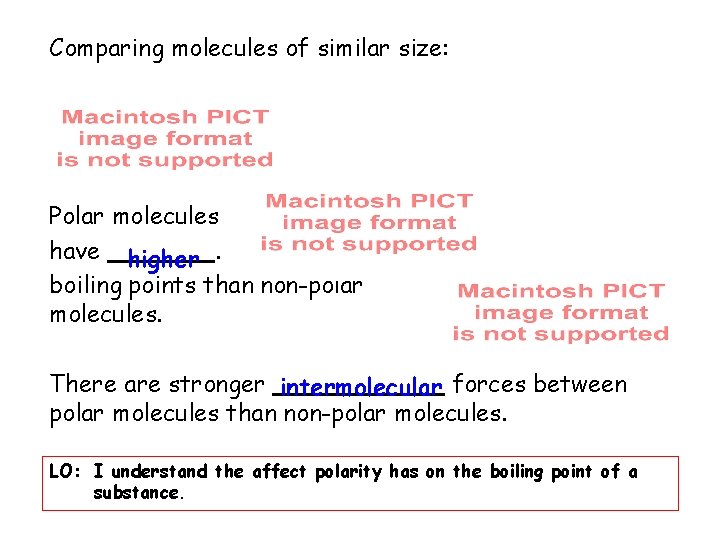
Comparing molecules of similar size: Polar molecules have higher. boiling points than non-polar molecules. There are stronger intermolecular forces between polar molecules than non-polar molecules. LO: I understand the affect polarity has on the boiling point of a substance.