Water The Facts Water is the most important













- Slides: 16


Water: The Facts ©Water is the most important molecule in living organisms. ©Water consists of 1 atom of oxygen combined with 2 atoms of hydrogen. ©Water makes up 70 to 95 percent of most organisms. H H

What are the characteristics of water? 1. Water is polar. 2. Water molecules attract other water molecules. This property is cohesion. 3. Water displays adhesion. 4. Water has a high specific heat. 5. Water expands when it freezes.

1. Water is Polar © A polar molecule has an unequal distribution of charge -each molecule has a positive end a negative end. © All polar substances are attracted to other polar substances.

Why is this polarity important? © Polar water molecules attract other water molecules as well as ions and other polar molecules. © Because of this attraction, water can dissolve many ionic compounds, such as salt and polar molecules like sugar. © Water is known as the universal solvent (almost anything can be dissolved by water)

©Solvent= what does the dissolving (water) ©Solute= what is being dissolved (sugar, salt) ©Solvent +solute=solution (sugar water) or (salt water)

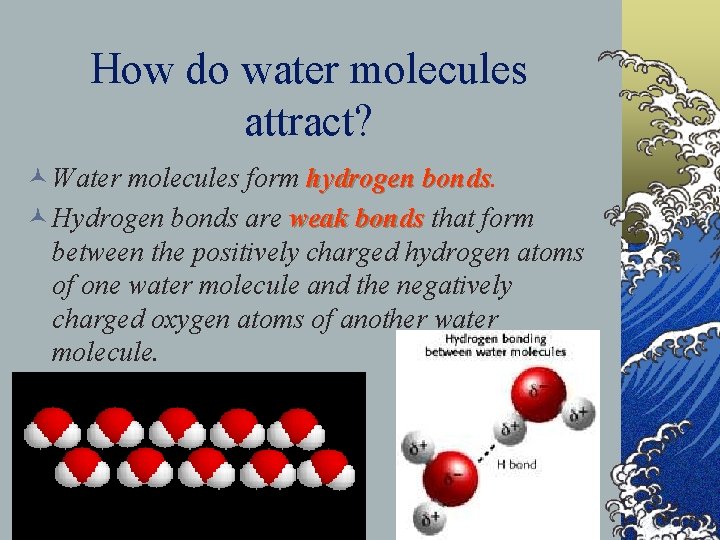
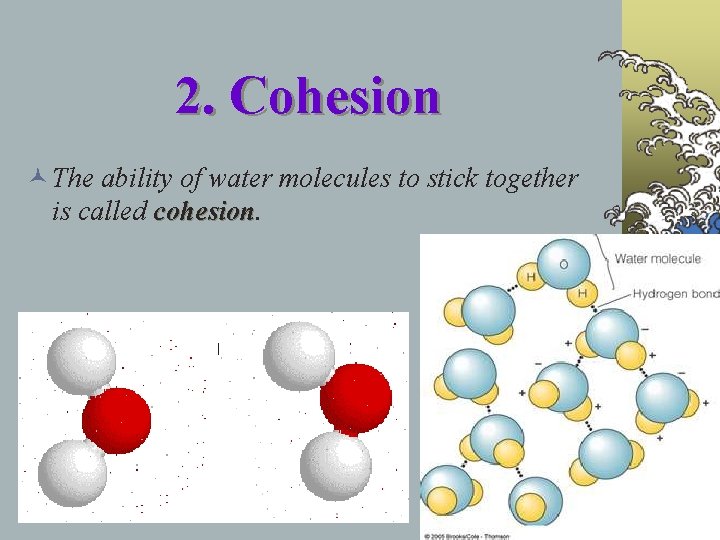
How do water molecules attract? © Water molecules form hydrogen bonds © Hydrogen bonds are weak bonds that form between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of another water molecule.

2. Cohesion © The ability of water molecules to stick together is called cohesion

Cohesion © Results in Surface tension (a measure of the strength of water’s surface) © Produces a surface film on water that allows insects to walk on the surface of water

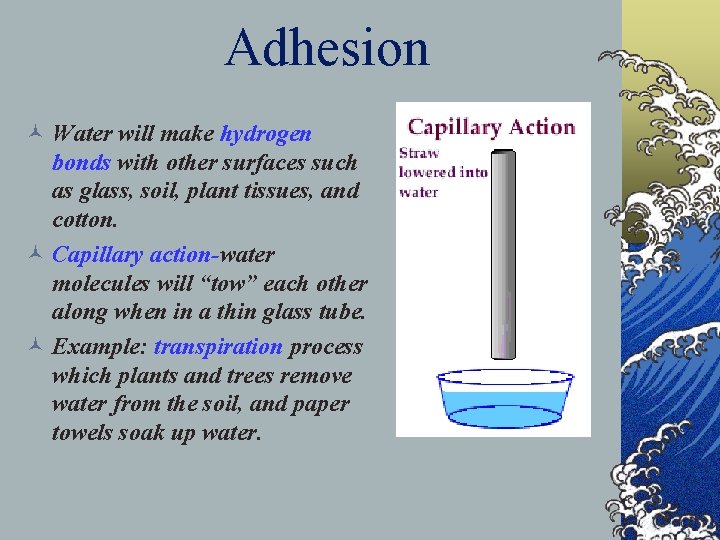
3. Adhesion © Water displays the property of adhesion which is the ability of one substance to cling to another. © Examples of adhesion include water’s ability to creep up thin tubes. Plants show this property when they send water from the soil up through their tissues.

Adhesion © Water will make hydrogen bonds with other surfaces such as glass, soil, plant tissues, and cotton. © Capillary action-water molecules will “tow” each other along when in a thin glass tube. © Example: transpiration process which plants and trees remove water from the soil, and paper towels soak up water.

4. High Specific Heat © Water resists temperature change, both for heating and cooling. © In order for water to evaporate, hydrogen bonds must be broken. © As water evaporates, it removes a lot of heat with it. © Water vapor forms a kind of global ‘‘blanket” which helps to keep the Earth warm.

5. Water expands when it freezes. © Because of this property, ice is less dense than liquid water. © This property can be seen when ice forms and floats on the surface of a pond.

Water expands when it freezes. ©Liquid water has hydrogen bonds that are constantly being broken and reformed. ©Frozen water forms a crystal-like lattice whereby molecules are set at fixed distances.

Water is Less Dense as a Solid! • Which is ice and which is water?

Water is Less Dense as a Solid! Water Ice
 Newspaper article format
Newspaper article format From most important to least important in writing
From most important to least important in writing Least important to most important
Least important to most important Water is one of the most important resource
Water is one of the most important resource Water and water and water water
Water and water and water water Middle colonies facts
Middle colonies facts Maine 13 colonies
Maine 13 colonies Middle colonies economy
Middle colonies economy Interesting facts about the middle colonies
Interesting facts about the middle colonies Native american important facts
Native american important facts Beowulf facts
Beowulf facts Multiplication facts and division facts
Multiplication facts and division facts 2 most important commandments
2 most important commandments Conclusion of salamanca statement
Conclusion of salamanca statement Agents of socialization sociology definition
Agents of socialization sociology definition Most important agent of socialization
Most important agent of socialization Different parts of body
Different parts of body