Properties of Water Polar molecule Cohesion and adhesion






- Slides: 12


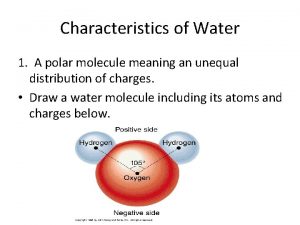
Properties of Water • Polar molecule • Cohesion and adhesion • High specific heat • Density – greatest at 4 o. C • Universal solvent of life

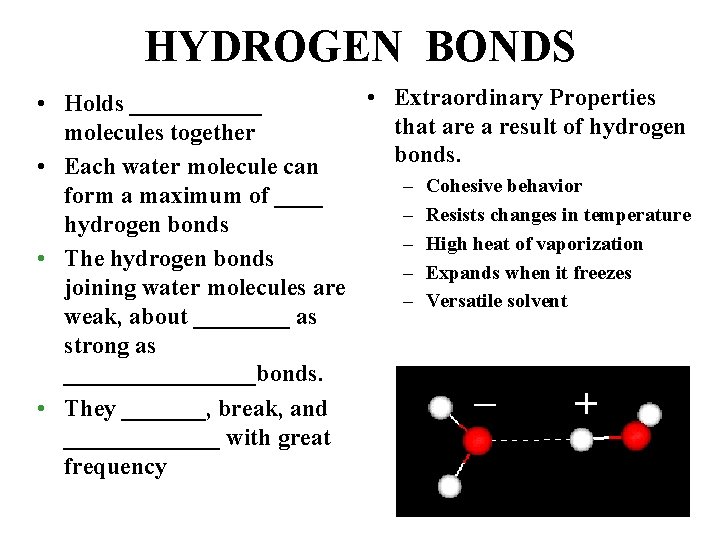
HYDROGEN BONDS • Extraordinary Properties • Holds ______ that are a result of hydrogen molecules together bonds. • Each water molecule can – Cohesive behavior form a maximum of ____ – Resists changes in temperature hydrogen bonds – High heat of vaporization • The hydrogen bonds – Expands when it freezes joining water molecules are – Versatile solvent weak, about ____ as strong as ________bonds. • They _______, break, and _______ with great frequency

Cohesion • Cohesion is responsible for the transport of the water column in plants • Cohesion among water molecules plays a key role in the transport of water against gravity in plants • Examples from the lab:

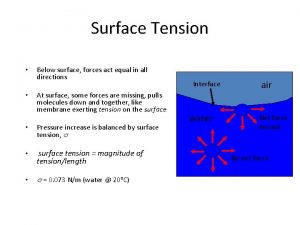
Surface Tension – Water has a greater surface tension than most other liquids because hydrogen bonds among surface water molecules resist stretching or breaking the surface. Examples from lab:

Adhesion • ______________ is related to adhesion and is the ability of a liquid to flow opposition to gravity as water “adheres” up a narrow column made of a polar substance, like glass or plastic. • Lab Examples:

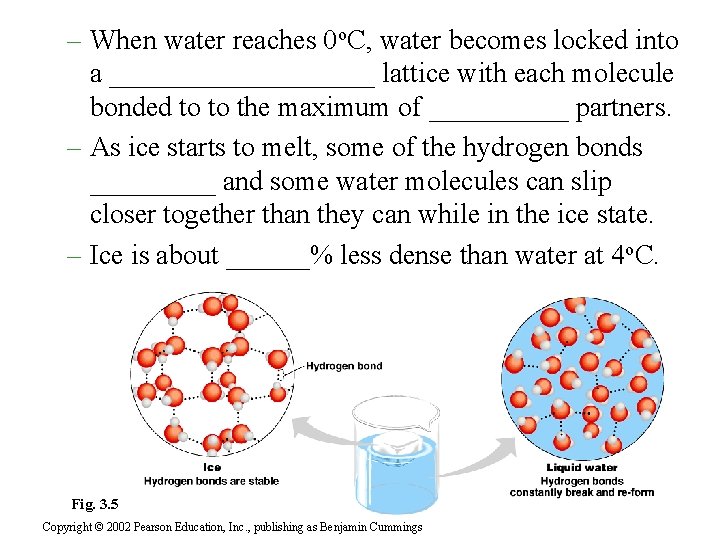
– When water reaches 0 o. C, water becomes locked into a __________ lattice with each molecule bonded to to the maximum of _____ partners. – As ice starts to melt, some of the hydrogen bonds _____ and some water molecules can slip closer together than they can while in the ice state. – Ice is about ______% less dense than water at 4 o. C. Fig. 3. 5 Copyright © 2002 Pearson Education, Inc. , publishing as Benjamin Cummings

Density of Water • Most dense at 4 o. C • ______ until 4 o. C • ______ from 4 o. C to 0 o. C The density of water: 1. Prevents water from freezing from the _______. 2. Ice forms on the surface first—the freezing of the water releases heat to the water below creating ___________. 3. Makes transition between season less abrupt.

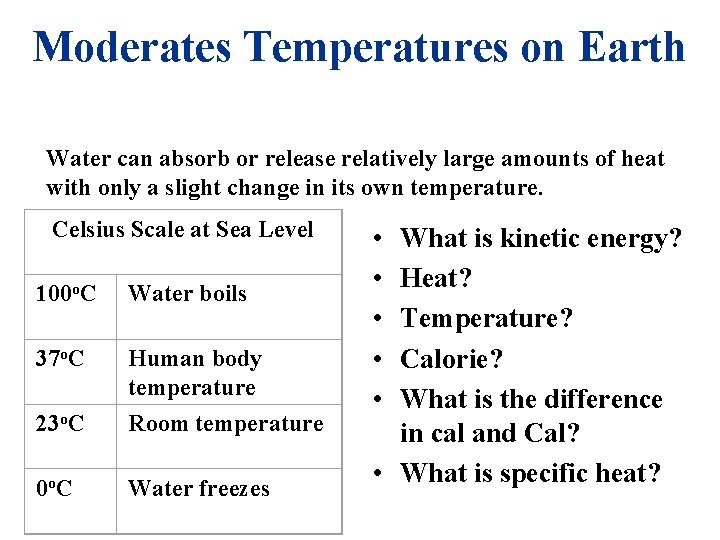
Moderates Temperatures on Earth Water can absorb or release relatively large amounts of heat with only a slight change in its own temperature. Celsius Scale at Sea Level 100 o. C Water boils 37 o. C 23 o. C Human body temperature Room temperature 0 o. C Water freezes • • • What is kinetic energy? Heat? Temperature? Calorie? What is the difference in cal and Cal? • What is specific heat?

Specific Heat: Water has a _______specific heat, meaning it takes a lot of energy to raise its temperature. 1. Prevention of temperature fluctuations that are outside the range suitable for life. 2. Coastal areas having a _____ climate 3. A stable marine environment

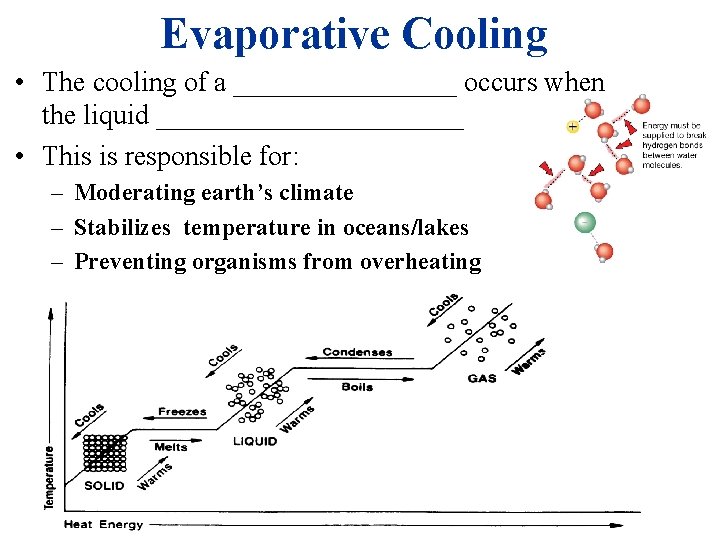
Evaporative Cooling • The cooling of a ________ occurs when the liquid ___________ • This is responsible for: – Moderating earth’s climate – Stabilizes temperature in oceans/lakes – Preventing organisms from overheating

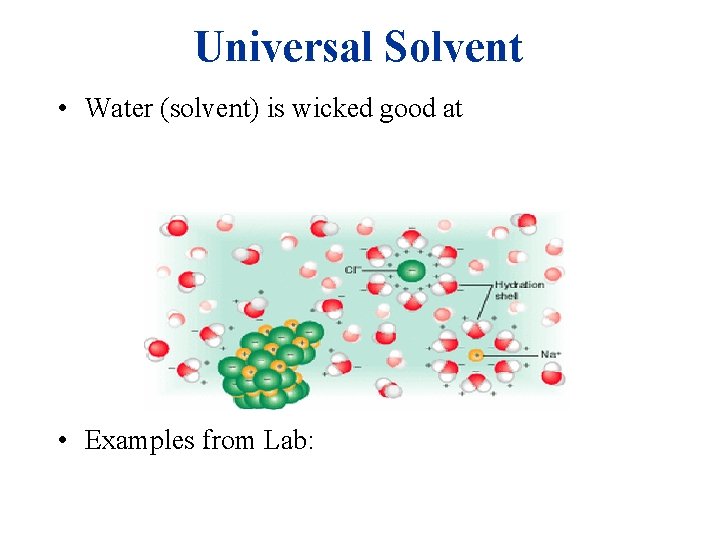
Universal Solvent • Water (solvent) is wicked good at • Examples from Lab: